-
(No explanation needed.) Which of the following are electrically neutral
(circle as many as apply)?
-
proton
-
neutron
-
electron
-
none of these
-
(No explanation needed.) What makes an element distinct?
-
the number of protons
-
the number of electrons
-
the number of neutrons
-
the total number of protons and neutrons
-
other
-
(No explanation needed.) A dam is thicker at the bottom than the top partly
because
-
water is denser at deeper levels
-
water pressure increases with depth
-
surface tension exists only on the surface of liquids
-
it looks better
-
none of these
-
(No explanation needed.) A one-ton blimp hovers in the air. The buoyant
force acting on it is
-
zero
-
less than one ton
-
one ton
-
more than one ton
-
need more information
-
(No explanation needed.) Objects that radiate well, also
-
absorb radiation well
-
reflect radiation well
-
both
-
neither
-
(No explanation needed.) Compared to the atoms in a newborn baby, the atoms
in the body of an elderly person are
-
newer
-
older
-
the same age
-
The volume of matter comes mostly from its
-
protons and neutrons
-
electrons
-
other
The protons and neutrons (which have almost all the mass of the
atom) are in the tiny nucleus. The electron orbits need the most
space.
-
The buoyant force on a rock is least when the rock is completely submerged
and
-
near the surface
-
near the bottom
-
halfway to the bottom
-
they're all the same
Pressure increases with depth, but the buoyant force depends only
on the weight of the displaced water. This is independent of depth.
-
In class I demonstrated Magdeburg Spheres that could not be pulled apart
when there was a vacuum inside. They have a surface area of about 30 in
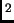 = 0.020 m
= 0.020 m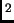 .
What is the force on the Magdeburg Spheres from atmospheric pressure? How
does this compare to the force that you can exert?
.
What is the force on the Magdeburg Spheres from atmospheric pressure? How
does this compare to the force that you can exert?
F = PA = 1.01 * 10^5 N/m^2 * 0.020 m^2 = 2.02 * 10^3 N
This is the weight of 202 kg = 440 lbs. Rather a lot!
-
A beaker is filled half-full with water and placed on a scale to measure
its weight. You lower a wooden rod partway into the water (without letting
go) so that it does not touch the sides or bottom of the beaker. The apparent
weight of the beaker (as measured by the scale)
-
increases
-
decreases
-
remains the same
-
depends on the size and density of the rod
-
need more information
We did this in class when I put my finger in the beaker!
The water exerts an upward buoyancy force on the rod. Therefore,
by Newton's 3rd law, the rod exerts a downward force on the water, increasing
the beaker's apparent weight.
An alternative explanation is that the rod displaces some water, increasing
the water level in the beaker. The increased water level increases
the pressure at the bottom of the beaker, increasing the downward force
exerted by the beaker plus water, increasing its apparent weight.
-
A large motorboat loaded with a barrel of water floats in a swimming pool.
If the water in the barrel is poured overboard (into the pool), the water
level in the pool will
-
rise
-
fall
-
remain unchanged
-
need more information
When the water is in the boat, it displaces its weight of water.
When the water is in the pool, the boat displaces less, but that is exactly
matched by the extra water in the pool. If you throw anything overboard
that floats (oris neutrally buoyant), the water level in the pool will
not change.
We did a similar experiment in class when I took a load of metal
out of a boat and dumped it overboard. Since the metal sank, the
water level in the tank decreased.
-
From how deep a hole can water be theoretically lifted by a vacuum pump
at sea level?
-
Less than 10 m deep
-
about 10 m deep
-
as deep as you want, just suck harder
-
need more information
Atmospheric pressure pushes the water up. Atmospheric pressure
can only push it up 10 m.
-
When a gas in a container is squeezed to half its volume (at constant temperature),
its pressure
-
quarters
-
halves
-
stays the same
-
doubles
-
quadruples
PV = constant (at constant temperature). If you halve
V, P must double.
-
During a very cold winter, water pipes sometimes burst. Why does this happen?
The fact that water changes volume with temperature does not really
matter here since the water is liquid and can flow to other parts of the
pipe. When the water freezes, it a) expands and b) can no longer
flow elsewhere. This means that it expands in one location.
The pipes cannot stretch that far and they burst. This is a serious
priblem in colder parts of the country.
-
An umbrella tends to move upwards on a windy day principally because
-
air gets trapped under the umbrella, warms, and rises
-
buoyancy increases with increasing wind speed
-
air pressure is reduced over the curved top surface
-
all of these
-
other
Bernoulli! The air under the umbrella might be warmer and
provide some buoyancy, but this is truly negligible.
-
Heat an iron plate with a small hole in it. The hole
-
gets bigger
-
gets smaller
-
remains the same size
We did this in class. The plate gets bigger without changing
its proportions. This means that the hole gets bigger too.
-
Aluminum has a specific heat capacity more than twice that of copper. Add
equal amounts of heat to equal masses of aluminum and copper. Which will
increase in temperature faster?
-
the aluminum
-
the copper
-
both the same
Aluminum has more thermal inertia. It takes more heat to make aluminum
change temperature than copper.
-
A can of air is sealed at atmospheric pressure and room temperature (20
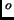 C or 70
C or 70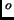 F). To double the pressure in the can, you must heat it to
F). To double the pressure in the can, you must heat it to
-
40
 C
C
-
140
 F
F
-
273
 C
C
-
313
 C
C
-
586
 C
C
You must double the temperature, but in Kelvin. 20 C = 293 K.
Double it to 586 K. Convert back: 586 K = 313 C.
-
A pot of clean snow and a pot of dirty snow are placed in the sunlight.
The snow to melt first will be the
-
clean snow
-
dirty snow
-
both the same
-
need more information
The dirty snow absorbs more sunlight and melts first.
-
The planet Earth loses heat mainly by
-
conduction
-
radiation
-
convection
-
all of these
-
none of these
The planet is surrounded by vacuum. This makes it really difficult
for it to lose heat by conduction or convection.
![]() Pa = 100,000 N/m
Pa = 100,000 N/m![]()
![]() C = -459
C = -459![]() F
F