Name: Honor Code Signature:
Physics 102 Exam 1
25 Feb 2005 Prof L. Weinstein
Please give a short explanation for all of your multiple-choice answers
(unless otherwise noted). Show your work for all numerical answers.
Useful numbers: Atmospheric pressure = 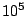 Pa = 100,000 N/m
Pa = 100,000 N/m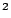
Absolute zero = 0 K = -273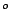 C = -459
C = -459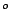 F
F
Density of mercury = 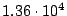 kg/m
kg/m
Density of water = 1000 kg/m
Density of air at room temperature = 1.2 kg/m
Density of helium at room temperature = 0.2 kg/m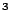
Acceleration of gravity at the Earth's surface: 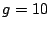 m/s
m/s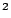
Acceleration of gravity at the Moon's surface: 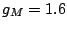 m/s
m/s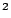
- Start with some neutral oxygen atoms, each of which have an atomic
weight of 16. If you add one proton to the nucleus of each atom, what will
you have (give the name of the resulting atom and its atomic weight)?
Oxygen (O) has atomic number 8 and atomic weight 16. If you add
one proton, you will have atomic number 9 and atomic weight 17. This
will be flourine-17.
- Start with some neutral oxygen atoms, each of which have an atomic
weight of 16. If you add one neutron to the nucleus of each atom, what will
you have (give the name of the resulting atom and its atomic weight)?
Oxygen (O) has atomic number 8 and atomic weight 16. If you add
one neutron, you will have atomic number 8 and atomic weight 17. This
will be oxygen-17.
- What is the pressure due to the water at the bottom of a 5 m (16 foot)
deep swimming pool on Earth?
p = dhg = (1000 kg/m^3)(5 m)(10 m/s^2) = 50,000 kg/m-s^2 = 5*10^4
N/m^2 = 5*10^4 Pa
- A car weighs 2000 pounds. The total area of its tires that is touching
the ground is 60 square inches. A racing bicycle and its rider weigh 200
pounds. The total area of its tires that is touching the ground is 2 square
inches. Which vehicle exerts more pressure on the ground?
- The car
- The bicycle
- both the same
- need more information
p = F/A
p_car = 2000 lb / 60 in^2 = 33 lb/in^2
p_bike = 200 lb / 2 in^2 = 100 lb/in^2
- In which configuration does the boat plus cargo displace more water,
when the cargo is carried on top of the boat (left picture) or when the cargo
is attached below the boat?
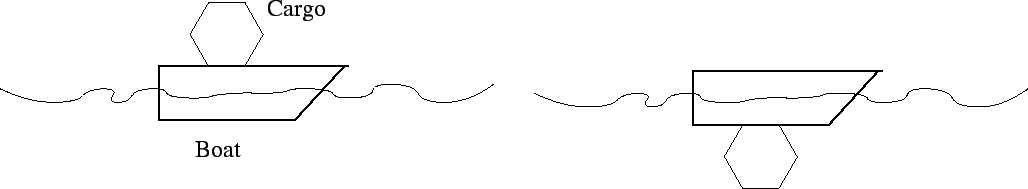
- Cargo on top
- Cargo underneath
- Both the same
- need more info
The cargo plus boat is floating in both cases and its weight is the
same in both cases. Therefore it displaces its weight of water in both
cases, which is the same.
- (no explanation needed) The Earth's climate was constant for thousands
of years until human release of large amounts of greenhouse gases started
global warming.
- True
- False (ice ages!)
- A rectangular barge in a lake is loaded so that 3 m (10 feet) of the
barge is below the water line and 1 m (3 ft) is above. If the barge was
then placed in a pool of mercury (see information at beginning of test),
then the barge would
- float higher (less than 3 m would be below the liquid line)
- float the same
- float lower (more than 3 m would be below the liquid line)
- sink
- need more information
Mercury is much denser than water. The barge will still displace
its weight of fluid, but it takes a much smaller volume of mercury to have
the same weight.
- A huge cargo ship weighs 100,000 tons (
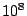 kg). The cargo ship enters a canal lock that is about 1 m wider than the
ship and 1 m longer than the ship. What is the minimum amount of water in
the canal lock needed to float the ship?
kg). The cargo ship enters a canal lock that is about 1 m wider than the
ship and 1 m longer than the ship. What is the minimum amount of water in
the canal lock needed to float the ship?
- less than 100,000 tons
- 100,000 tons
- more than 100,000 tons
- need more information
We did this in class with the Battleship in the Bathtub. You only
need enough water remaining in the lock to go all around the ship. Yes,
the ship must displace 100,000 tons of water. However, the water displaced
is NOT THERE ANYMORE. (In the case of the battleship and the bathtub,
the displaced water is on the bathroom floor and clearly not helping to support
the ship.) I gave half credit for showing that the ship must displace
100,000 tons of water.
- How large a helium bag (in m
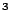 ) would you need to lift a 60-kg (130 lb) person? Ignore the mass of the
bag itself.
) would you need to lift a 60-kg (130 lb) person? Ignore the mass of the
bag itself.
The weight of the displaced air provides the buoyant force. You
must displace enough air to provide the bouyant force to lift you plus the
helium. If the volume of the bag is V, then the weight of you plus the helium
is
(m + d_helium * V) * g
and this must be equal to the weight of the displaced air = (d_air * V) *
g so that
(60 kg + (0.2 kg/m^3 * V) = (1.2 kg/m^3 * V) and thus
60 kg = V * (1.2 kg/m^3 - 0.2 kg/m^3) = V * 1 kg/m^3 or
V = 60 m^3
- Extra credit (since the problem was not cler enough) You are
looking down on a piece of a horizontal pipe system that is filled with water.
Water is flowing from North to South in the large pipe. Which direction
is water most likely to flow in the smaller pipe?
= 1.2in 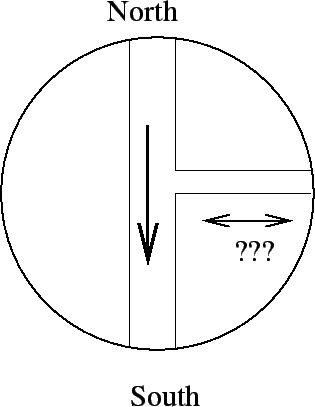
- from left to right
- from right to left - Bernoulli
- it will not flow
- need more information
- (No explanation needed) When the temperature outside is 35
 F and the wind speed is 20 mph, it feels as cold as it would if it was
24
F and the wind speed is 20 mph, it feels as cold as it would if it was
24  F with no wind. Will water freeze under these conditions (35
F with no wind. Will water freeze under these conditions (35  F and 20 mph)? (Assume that it is daytime but completely cloudy.)
F and 20 mph)? (Assume that it is daytime but completely cloudy.)
- Yes
- No - water does not feel wind chill since it does not generate its
own heat
- Need more information
- Two nested drinking glasses are stuck together. We want to separate
them by running water over them. The best way to do this would be
= 1.in 
- run hot water on the inner one and cold water on the outer one
- run cold water on the inner one and hot water on the outer one -
make the inner one contract and the outer one expand
- run cold water on both
- run hot water on both
- A 1000-W microwave oven can transfer 1000 J every second to the food
in it. Start with a 0.5-kg (1-pound) potato and 0.5 kg (0.5 liter) of water
at room temperature. If you put each of them in their own 1000-W microwave
oven at high power, which will have a higher temperature after two minutes?
- the potato
- the water
- both the same
- need more information
Water has a higher heat capacity than just about anything else so the
same amount of energy put into water will not raise its temperature as much
as the potato.
- (No explanation needed) A hot object is placed touching a colder object.
Which of the following is true?
- The hot object will lose as much temperature as the cold object gains
- The hot object will lose as much heat as the cold object gains -
energy is conserved, not temperature
- both of the above
- neither of the above
- (no explanation needed) Electric socks have batteries and heating
coils in the sock. How is the heat transferred to your feet?
- conduction - the socks are touching your feet
- convection
- radiation
- thermal expansion
- other
- (no explanation needed) Some people blow warm air on their hands to
warm them up in the winter. How is the heat transferred to your hands?
- conduction
- convection - the warm air is moving from your mouth to your hands
- radiation
- thermal expansion
- other
- (no explanation needed) A 17th century bread oven was a brick box
with very thick walls. The bakers would put wood in the oven and light a
fire. When the fire had burned for a few hours, the walls of the oven would
be very hot. They would then remove the fire and put in the bread to bake
on a rack. How was heat transferred to the baking bread?
- conduction
- convection
- radiation - the hot walls of the oven radiate heat. The hot
air in the oven is a poor heat conductor (but I gave partial credit for conduction)
- thermal expansion
- other
- The temperature in your room is 10
 C (50
C (50  F). You ask your roommate to double the temperature. To what temperature
should your roommate set the thermostat in order to comply with your request?
F). You ask your roommate to double the temperature. To what temperature
should your roommate set the thermostat in order to comply with your request?
measure from the floor! T = 10 C + 273 = 283 K
2T = 566 K = 293 C
This is why physicists make dangerous roommates!
- A heat engine runs an electrical generator. The boiler heats steam
to a temperature of 500
 C, the steam turns a turbine to generate electricity, and then a condenser
cools the remaining steam to a temperature of 80
C, the steam turns a turbine to generate electricity, and then a condenser
cools the remaining steam to a temperature of 80 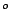 C. What is the maximum thermal efficiency of the generator?
C. What is the maximum thermal efficiency of the generator?
measure from the floor!
effi = (T_hot - T_cold)/T_hot = ((500 + 273) - (80 + 273)) / (500 +
273)
effi = 420 / 773 = 0.54
- (No explanation needed) In the previous problem, what could you do
to increase the maximum thermal efficiency of the generator? Circle all that
apply.
- decrease the boiler temperature
- decrease the condenser temperature
- increase the boiler temperature
- increase the condenser temperature
- make the boiler bigger
- make the condenser bigger
2005-03-01
![]() Pa = 100,000 N/m
Pa = 100,000 N/m![]()
![]() C = -459
C = -459![]() F
F ![]() kg/m
kg/m![]()
![]()
![]()
![]()
![]() m/s
m/s![]()
![]() m/s
m/s![]()


