
Read the pages indicated by A-3 and study behavioral objectives 3 and 4. The foundations of our current model of atomic structure had its very beginnings around 460 B.C. with the early Greeks. In this period, Greek philosophers, such as Democritus, considered many theories about the composition of matter. Democritus and others who followed his school of thought, believed that matter was composed of very small particles that were infinite in number and could not be divided or cut into smaller pieces. To these particles the Greek word atomos was given, meaning indivisible or uncutable.
It took several centuries for this atomic model to develop into one that we accept as reflective of the true structure of the atom. Earnest Rutherford, around the turn of the twentieth century, established a model of the atom consisting of a core, called the nucleus , which carries a positive charge and the bulk of the mass, and a very small negatively charged particle called an electron which orbits the nucleus. The electron does its best to spread its charge around the nucleus and forms in the process what is called an electron cloud. The result of the positive charge of the nucleus and the negative charge of the electron is the electron being held captive by the attraction of the nuclear positive charge. The particle that holds this positive charge in the nucleus is called the proton. An atom may have one or more protons in its nucleus and one or more electrons in orbit around the nucleus. If there is one electron for every proton in the nucleus the atom is electrically neutral. One other particle that can reside in the nucleus has a neutral charge. The name of this particle comes from its charge. It is called a neutron, of which an atom can have from none to many.
These particles that comprise the nucleus are called elementary particles. Besides having a charge, these elementary particles also have mass. The mass of a particle is referred to as its mass number. The mass of a proton is extremely small, 1.67 x 10-24 grams. This number is so small that it is easier to use a conversion factor when measuring the mass of atomic particles. One atomic mass unit (amu) is equal to 1.660 x 10-24 grams. Therefore the proton is considered to have a mass of 1 amu, the neutron is slightly heavier than the proton, 1.675 x 10-24 grams. When you convert this unit of measurement into atomic mass units is also comes out to be approximately 1 amu. The electron whose mass is 1/1837 the mass of the proton or 9.11 x 10 -28 grams which converts into .000549 amu is so light compared to the mass of a proton or neutrons that it is usually not considered when evaluating the total mass of an atom.
Fill in the table below:
| Subatomic particles | Charge | Mass (amu) |
| protons | ||
| neutrons | ||
| electrons |
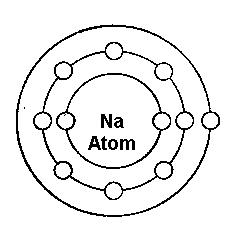
Sodium Atom - protons and neutrons in nucleus, electrons in orbits.
1. The center of the atom is called the_________?
- a. nuclear pit
- b. coreolus
- c. nucleus
2. The proton is found ________?
- a. orbiting the nucleus
- b. in the nucleus
- c. outside the nucleus
3. The electron is found_______?
- a. in combination with the proton at the center of the atom
- b. orbiting the nucleus forming the electron cloud
- c. in combination with the neutron at the atomic core
4. The elementary particles, proton, electron and neutron have mass and charge values. What is the mass of the proton?
- a. 1.5
- b. 1
- c. 0.5
5. The proton, which is found in the nucleus, has a charge value of what?
- a. -1
- b. +2
- c. +1
6. The electron, which orbits the nucleus, has a charge value of what?
- a. -1
- b. +1
- c. -0.5
7. The neutron, which can be found in the nucleus, has a mass value of what?
- a. 0 amu
- b. 1 g
- c. 1 amu
8. The neutron carries a charge value of what?
- a. 0 or neutral
- b. -1
- c. +1
9. The electron has a mass of what?
- a. +1
- b. 0
- c. almost 0, about 1/1837 the mass of the proton
10. The protons, and neutrons if present, reside in the nucleus of the atom. Since the protons have a positive charge and the neutrons, as the name suggests, have a neutral charge (no charge at all), the net over all charge of the nucleus is what?
- a. zero
- b. negative
- c. positive
The electron is the particle that comprises the electron cloud around the nucleus. The charge of the electron cloud is therefore negative. This negative charge balances out the positive charge of the nucleus in a normal uncharged atom. There is one electron for every proton in the nucleus of a normal uncharged atom.
11. Oxygen, which has 8 protons in its nucleus, would need how many electrons in order to be normal and uncharged?
- a. 16
- b. 8
- c. 4
A model of the atom has now been established. We have a positively charged core called the nucleus which consists of a one or more protons, and may have from none to many neutrons. Around the atom we have one or more negatively charged electrons orbiting the nucleus. When the number of electrons orbiting the nucleus equals the number of protons in the nucleus the atom has a neutral electrical charge. If the neutral atom loses an electron, the atoms electrical charge becomes positive by +1. This positive charge increases by an additional +1 for every electron lost for a corresponding proton in the nucleus. For example, if the atom had 8 protons and 8 electrons in its neutral state, and lost 3 of those electrons, the atom would assume a +3 charge.
12. What would be the charge of this atom if it lost only 2 electrons?
- a. -2
- b. +2
- c. +6
The atom is the smallest part of an element. There are about 105 known elements. Of these only about 90 are found in the earth's crust and of these only about 20 are found in living organisms. Ninety-nine percent of most living organisms consist of only six of these elements. These are:
| Element | Man | Alfalfa | Bacterium |
| Carbon | 09.37 | 11.34 | 12.14 |
| Hydrogen | 09.31 | 08.72 | 09.94 |
| Nitrogen | 05.14 | 00.83 | 03.04 |
| Oxygen | 62.81 | 77.90 | 73.68 |
| Phosphorus | 00.63 | 00.71 | 00.60 |
| Sulfur | 00.64 | 00/10 | 00.32 |
| Total | 97.90 | 99.60 | 99.72 |
For example, the element oxygen, which has 8 protons and 8 neutrons, has an atomic number of 8, and an atomic weight of 16 amu.
If we consider the element potassium, which has 19 protons and 20 neutrons, the atomic number is 19 and the atomic weight is approximately 39 amu.
13. What would be the atomic weight of carbon which has 6 protons and 6 neutrons?
- a. 36 amu
- b. 12 g
- c. 6 amu
- d. 12 amu
14. What would be the atomic number of
carbon (atomic mass of 12 amu
containing six protons and six neutrons)?
- a. 6
- b. 12
- c. 36
In your notes draw a figure showing the parts of an atom and write definitions for the terminology used above when describing an atom.
- Do atoms have to contain neutrons?
- Do all atoms of the same element have to contain the same number of neutrons and protons?
- Do all atoms of the same element have the same mass?
- Do the number of protons and neutrons in an atom have to be the same?
To go to the next home page answer the following question correctly.
15. The element Ar (argon) has an atomic mass of 39.9 and atomic number of 18. How many neutrons does an atom of argon have?
For information on how to use this page, go to How to Use This Site.Created by the Center for Learning Technologies, Academic Technology Services.
Last modified October 22, 1997.