
Proteins: Polymers of amino acids
Study behavioral objectives 25, 27, 29 and 31. Read the pages in the text indicated by A-10 concerning protein. Proteins are macromolecules which contain the elements carbon, hydrogen, oxygen, nitrogen and may contain sulfur. Proteins are polymers of amino acids. Proteins are extremely important in cell structure and function. Among the cellular functions of proteins are structural support, protection, catalysis transport, defense, regulation, and movement. Of particular importance are the catalytic proteins called enzymes.
Proteins are polymers of amino acids. There are approximately twenty different types of amino acids. Proteins can vary in length from two amino acids to a chain of millions of amino acids (polypeptide).
Two amino acids synthesized together = dipeptide
Three amino acids synthesized together = tripeptide
Four to billions amino acids synthesized together = polypeptides or protein
Examples of protein are hair, claws of reptiles, birds and mammals, egg albumin, gelatin, etc.
In order to understand proteins let us examine the general structure of amino acids.
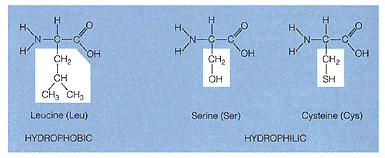
three amino acids
Amino Acid Structure: Amino acids consist of a carbon skeleton consisting of one carbon with three attached groups. Carboxyl group - this in the functional group studies earlier. -(-COOH) The carboxyl group will be on one end of the amino acid molecules and will provide the -OH group to form water during condensation synthesis of amino acids to form protein. Identify this group in the figure above. Amine group - this is the functional group studied earlier. (-NH ) The amino group will be on the opposite end of the amino acid as the carboxyl group and will provide the -H group to form water during the condensation of synthesis of amino acids to form protein. Identify this group in the figure above. "R" group - this group may be any of the twenty different groups of atoms which provide the protein with its characteristics. This group in the figure above is located in the boxes. Your text will show a few of these twenty different types. These groups vary is structure, thus there are twenty different types of amino acids.

amino acids
The above figure contains the structural formula for three amino acids. Fine the amino group, carboxyl group and the "R" group. The "R" group is outlined in white. In nature their are approximately twenty different types of amino acids based on the "R" groups. These "R" groups produce the chemical properties of the amino acids and the proteins which they makeup.
17. In your notes draw a figure showing the general structural formula of an amino acid.
Press here to check your answer.
Amino acids are hooked together to forming covalent bonds between the
amino acid monomers. This is accomplished as discussed earlier by condensation
synthesis. Let us review condensation synthesis: During the process of
condensation synthesis one water molecule between each monomer will be
removed. Each monomer contributes part of the water molecule that is released;
one monomer loses a hydroxyl group (OH), and the other loses a hydrogen
(H). Both monomers, having each lost a covalent partner, now bond covalently
with each other. This time the OH is removed from the carboxyl group of
one amino acid and the H is removed from the amino group of a second acid
amino acid. Trace the events described above using the figure below.
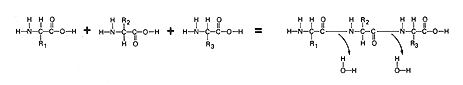
Note: The "R" groups are indicated by "R1, R2, etc". They are not drawn out.
18. How many water molecules will be formed when four amino acids condense to form a protein molecules?
Press here to check your answer.
In your notes using structural formulas write a reaction hooking three
amino acids together during a synthesis condensation reaction.
Note that the bond which forms during the synthesis of two or more amino acids contains a C-N-C bond since water is cleaved between an amino group and an organic acid group. This bond is called a peptide bond. It exists only in proteins. Locate the two peptide bonds in the protein molecule below.

19. How many peptide bonds does a tripeptide contain? Press here
to check your answer.
Not only do different protein contain different lengths of amino acids,
but the amino acids can be arranged in any order. Therefore their is almost
an infinite number of proteins.
The precise sequence of amino acids in a protein is called its primary structure.
20. What is the primary structure of the following protein?
ser-gly-gly-glut-arg-thre
Press here to check answer.
21. Protein can also be hydrolyzed. If you had eggs for breakfast this
morning the protein of the egg albumin (egg white) is in your large intestine.
This protein is now being hydrolyzed (digested) into what products?
Press here to check your answer.
Nucleic Acids: DNA/RNA
Read the pages in the text indicated by A-10
and reexamine behavior
objectives 27. Nucleic acids are macromolecules which contain the elements
carbon, hydrogen, oxygen, nitrogen and phosphorus. Like protein and starch
these are linear polymers. The monomers which are covalently bonded are
called nucleotides. Examine the figures of nucleotides and nucleic
acids which are found in your readings. Nucleotides are made of the following
parts:
five carbon sugar - ribose or deoxyribose - these sugars are present in the form of a five sided ring.
phosphate- this is the functional group discussed earlier
nitrogen-containing base - there are five different types of bases found in nucleic acids
Examine the figure below and locate each of the nucleotide parts.
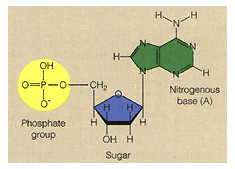
nucleotide
These nucleotide can be hooked together by condensation synthesis forming a polymer of nucleic acids. When these monomers link by condensation synthesis the phosphate group links to the sugar group forming a long polymer of P-S-P-S-P-S-P-S with the nitrogenous base sticking above the polymer.
There are two type of nucleic acids DNA and RNA. Chemical differences between these two nucleic acids will be discussed in miniunit Delta.
DNA- macromolecules are used for gene expression and the control of all cellular activities by directing protein synthesis. The structure and function of this molecule will be studied in miniunit Delta.
RNA - macromolecules are used to aid the DNA molecules during gene expression. They are also act as messengers and transfer molecules during protein synthesis.
Nucleic acids can also be hydrolyzed.
22. The hydrolysis of nucleic acids form:
a) amino acids
b) fatty acids
c) fructose
d) nucleotides
Press here to check your answer.
This is the end of miniunit Alpha. You may now go back to the
Miniunit Alpha Lessons Page and
take the practice test.
For information on how to use this page, go to How to Use This Site.
Created by the Center for Learning Technologies, Academic Technology Services.
Last modified October 22, 1997.