 glucose (six sided ring)
glucose (six sided ring)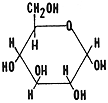
Press here to go back to home page. press
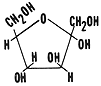
2. These are isomers. Glucose and fructose are isomers in that
they have the same molecular formula but different structural formulas.
Glucose is a six sided ring and fructose is a five sided ring. Study the
structural formulas for these two molecules. Draw the shorthand notation
for these two structural formulas in your notes.
fructose (five sided ring)  glucose (six sided ring)
glucose (six sided ring)
Press here to go back to home page. press
3. The correct answer is "b". These two disaccharides have the same
molecular formula but different structural formulas.
Press here to go back to home page. press
4. For each maltose sugar hydrolyzed two glucose molecules would be
released and one water molecules would be required.
hydrolysis 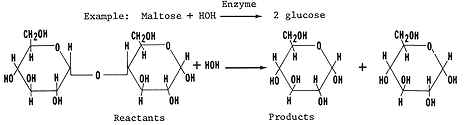
Press here to go back to home page. press
5. The sucrose in your stomach is being hydrolyzed into one glucose
and one fructose monomer per sucrose molecule.
Press here to go back to home page. press
Press here to go back to home page. press
7. The reason is that one water molecule is removed between these two
monomers; therefore, the disaccharide formed will contain two less hydrogen
atoms and one less oxygen.
Press here to go back to home page. press
8. The correct answer is "b". Starch is a polymer of many (millions)
of glucose monomers.
Press here to go back to home page. press
9. The starch in bread is broken down into glucose. You are presently
absorbing glucose into you blood stream.
Press here to go back to home page. press
10. Does cooking mix with water? Plant oils are triglycerides
or lipids. They are all nonpolar.
Press here to go back to home page. press
11. The correct answer is three. A water molecule is required to form
a bond between each of the three alcohols on a glycerol molecule to attach
to a fatty acid molecule.
Press here to go back to home page. press
12. The butter in you stomach is being digested (hydrolyzed) into three
fatty acids and one glycerol molecule for each triglyceride.
Press here to go back to home page. press
13. The part of the phospholipid molecule soluble in water (polar)
would face inwards to dissolve in water phase and the part of the
molecules which in nonpolar would face up out of the water or mix with
other nonpolar parts of the lipid forming a bilayer.
Press here to go back to home page. press
14. No. They are not made of repeating monomers. They consist of two
type of monomers (glycerol and fatty acids) not in the form of a chain.
Press here to go back to home page. press
15. No. steroids are lipids which are not made of monomers; therefore,
they can not be hydrolyzed.
Press here to go back to home page. press
17. Your structural formula should consist of a single
carbon atom (four bonds) with an amine group attached to one end (first
bond), and organic acid to the opposite end (second bond), a hydrogen attached
to the third carbon bond and an "R" group which can be any of twenty structures
on the fourth carbon bond.
"R"
|
HN-C-COOH
|
H
Press here to go back to home page. press
18. Four amino acids attached by peptide bonds would contain only three
of these peptide bonds. During hydrolysis of this protein only three
water molecules would split out.
Press here to go back to home page. press
19. A tripeptide consists of three amino acids and in has only two
peptide bonds. Draw a tripeptide molecule in your notes and count
the number of C-N linkages.
Press here to go back to home page. press
20. The primary structure is simply the order of amino acids.
The primary structure is: serine bonded to a glycine bonded to another
glycine bonded to a glutamine bonded to an arginine bonded to a threonine.
Press here to go back to home page. press
21. Egg albumin consists of protein. It is digested in your large intestine
into its monomers, individual amino acids.
Press here to go back to home page. press
22. The correct answer is "d". Nucleic acids DNA,RNA) are polymers
of nucleic acids.
Press here to go back to home page. press
.
220.
Press here to go back to home page.
21.
Press here to go back to home page.
22.
Press here to go back to home page.
Press here to go back to home page.
Press here to go back to home page.