
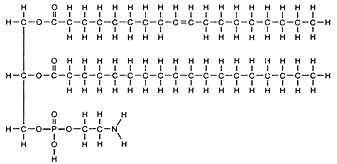
Note: Locate the hydrophilic phospholipid part of the fat molecules
above.
Locate the hydrophobic
portion of the phospholipid.
Read the pages in the text describing phospholipid structure.
In biological membrane which you will study in miniunit
Beta phospholipids line up in such a way that the nonpolar, hydrophobic
tails pack tightly together to form the interior of the membrane, and the
phosphate containing "heads" face outward (some on either side, where
they interact with water. The phospholipid forms a bilayer, a sheet
two molecules thick. Only nonpolar molecules will be allowed to pass
through the lipid portion of this barrier. Polar molecules
will have to pass through by using special protein molecules or pores.
Click here to go back to the home page. click
Created by the Center for Learning Technologies, Academic Technology Services.
Last modified October 22, 1997.