In miniunit Alpha you learned that carbohydrates (glucose) are molecules that living systems use to store biological energy. In miniunit Beta you were introduced to plant and animal cells and their organelles, what macromolecular these organelles consist of and their function in the cell. For a cell to synthesize these organelles biological energy is required. You also learned in miniunit Beta that for cells to move molecules across the cell membrane (active transport) biological energy is required. In miniunit Gamma you studied how the nucleus functions during cell division mitosis and meiosis. During miniunit Delta you examined how genetic traits are passed from one generation to another and how the nucleus controls cell activities through protein synthesis. To perform these various cell processes a cell must have a source of energy. In Epsilon, you will learn how a cell aquires its energy to perform these various cell processes. The first part of Epsilon will deal with how cells slowly break down energy rich molecules into energy poor molecules converting the energy released into biological energy through chemical processes called cellular respiration. The second part deals with how energy rich molecules are synthesized from energy poor molecules through a process called photosynthesis.
Metabolism is the sum of all chemical reactions that occur within
a cell (respiration, photosynthesis, digestion, organic molecule synthesis,
etc.). Metabolism may be divided into two parts - catabolism and anabolism.
Catabolism is the breaking down of complex (energy rich) molecules to simpler
products with an accompanying release of energy. Anabolism is the building
up of complex (energy rich) molecules from simpler ones requiring an input
of energy. Examine the various examples to the right and indicate if they
are a type of anabolism or catabolism. Study pages indicated by E-1
in the text and write a paragraph on behavioral objective 1.
Study behavioral objectives 2-5. Read the pages in the text indicated by E-2.
Living things are both complex and dynamic, and as such it is one of the most interesting and challenging objects for scientific study. One excellent method for studying and understanding complex physical phenomena has been obtained from thermodynamics, a branch of science which examines selected features of the physical world from unique perspectives. Thermodynamic study is a tool through which we can learn much about living things. You will study this branch of study to better understand energies, respiration and photosynthesis.
In thermodynamics, as in all areas of science, the purpose is to learn about the behavior of matter. Thermodynamics provides a useful scientific language for the study of matter and its behavior.
What makes matter behave as it does? Suppose you were to hold a box of matches upside down over a table and slide the box open. How would the matches (matter) behave? (Click one.)
- a. They would go upward through the air.
- b. They would stay suspended in the box.
- c. They would fall to the surface of the table.
- a. straight row
- b. regular pattern
- c. disorganized heap
- a. predictable
- b. strange
- c. improbable

You would indeed be surprised if the matches went some other direction, like upwards or sideways, when you opened the box. You would also be surprised if the matches arranged themselves into a predictable pattern on the table.
The matches move from the unstable (organized) position of being in an open box to a relatively stable (disorganized) position on the table. Unless some new object or force acts upon the matches, you would expect them to (click one):
The behavior of all matter, like that of the matches, is dependable, even predictable, because it is governed by physical laws. From the study of thermodynamics we can learn some of the rules which govern the predictable behaviors of matter.Scientists learn about the behavior of matter by conducting experiments. Since it is impossible to examine all matter, scientists must isolate specific segments of the physical universe for their study. In the language of thermodynamics, the part of the universe under investigation in an experiment is called a system. A system is simply the small part of the universe which has been defined for the purpose of experimentation.
Most of thermodynamic study involves measuring changes in systems and examining the interactions that take place between systems and their surroundings. It is through the study of these changes and interactions that scientists have learned some of the rules which govern (click one):
- a. science
- b. matter's behavior
- c. experimentation
Since energy is a better known concept, we will study some of its aspects first and deal with entropy later.
A system can absorb energy and release energy. Hot coffee (the system) releases heat energy to the cup and surrounding air as it cools. The cooling process is the transfer of energy from the system to the surroundings. Leaves of a plant (the system) absorb light energy from the sun. Absorption of an amount of energy by a system means that the energy moves (click one):
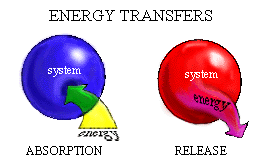
Both energy absorption and energy release by a system involve the transfer of energy across the defined boundaries of the system. Such energy transfer processes change the energy content of the system.
When a leaf absorbs energy from the sun, the energy content of the leaf "system" rises. As hot coffee in a cup cools, the energy content of the coffee "system" (click one):
- a. is raised
- b. is lowered
- c. stays the same
If a system obtains energy from its surroundings, the surroundings will lose (click one):
- a. some of the energy gained by the system
- b. all of the energy gained by the system
- c. none of the energy gained by the system
- a. The amount of energy lost by the coffee equals the amount of energy gained by the surroundings.
- b. The amount of energy lost by the coffee equals the amount of energy gained by the universe.
- c. The amount of energy lost by the coffee is greater than the amount of energy gained by the surroundings.
The course of all physical events is such that the energy of the universe is conserved.
Living systems also behave according to the First Law of Thermodynamics just as non-living systems. It is often said that living systems "use" or "consume energy". You should understand that this does not mean that the energy vanishes or disappears. Living matter, like non-living matter, can absorb, release, and transform energy, but it cannot create or destroy it. Energy "used" by a living system is simply energy that is incorporated into the living system in some form. In the case of a plant absorbing light energy from the sun, the energy becomes part of the plant in the form of chemical energy. The actual energy from the sun is neither created nor destroyed.
In the physical world, the natural tendency of all matter is toward becoming more stable. This means that matter in a system, whether living or non-living, tends to adjust itself to attain greater stability.
In energy terms, a stable system an be thought of as one which is low in potential energy. Matches fall toward the earth in response to the attractive force, of gravity. This movement to greater stability is a move toward (click one):
- a. lower potential energy for the system
- b. higher potential energy for the system
- c. energy conservation for the system
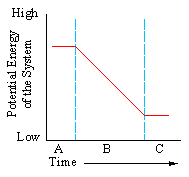 |
|
- a. uniform levels
- b. higher levels
- c. lower levels
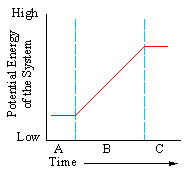 |
|
- a. The system would adjust to lower potential energy.
- b. The system would increase its potential energy.
- c. The system would become less stable.
You have seen that energy is an important factor in the behavior of matter. Entropy, while not as familiar to most people as energy, is also a physical property closely linked with matter's behavior.

Entropy is difficult to define because of its many aspects, but it
appears to be related to the organization of matter in a given system.
An increase in the entropy of a system is evidenced as an increase in disorganization
and disarray. For our purposes, then, we can consider entropy to mean (click
one):
- a. the degree of order in a system
- b. the degree of disorder in a system The Second Law of Thermodynamics states that the entropy of the universe is always increasing. In other words, matter in the universe is becoming more and more (click one):
- a. ordered
- b. disorganized
- c. plentiful
Just as systems adjust to gain stability, they appear just as naturally to (click one):
It is no surprise, then, that matches dropped from an open box land in disarray. Their tendency is toward both (click one): To arrange the matches into a straight row, or to make a desk neat, or to clean up your room requires an expenditure of energy on your part.The tendency of the entropy in a system to increase may be counteracted by using energy from outside the system. Let us use your bedroom as an example. Is your bedroom stable or unstable. During the weekend you organize the room -books organized on desk, clothes on hanger in closet, ect. Is this room stable or unstable? It is very unstable. During the week the tendency will be for the room to become disorganized. Unless you add energy in the form of work, this room will become disorganized which is the more stable condition. It takes energy to reverse the natural processes which decrease the order of systems. You must work (expend energy) to halt, even in any small fashion, the (click one):
Without a constant input of energy, living things respond to the Second Law and entropy takes over -- the living thing falls into disorganization and death. Living matter is as subject as non-living matter to the entropy trend of the universe. When a creature can no longer obtain energy to fight the entropy trend, it starts to become disorganized. Without high organization, life processes cannot occur, so the creature dies. Energy is necessary to keep the decay processes from occurring.In the face of the natural tendencies toward stability and disorganization, living matter is able to maintain dynamic processes and a high degree of organization. Even the smallest and simplest unit of living matter-the cells a tremendously dynamic and complex system. In order to operate and live, the cell must maintain a fantastic degree of organization.
How does the cell preserve its organization? (Click one.)
In accordance with the First Law of Thermodynamics (conservation of energy), the cell cannot create energy. It can, however, use energy. It can absorb, release, and transform energy. Through just such procedures the cell can maintain the high degree of organization required for survival.Cells have acquired the mechanisms for conducting the necessary transformations of energy. We can say that living matter creates order at the expense of energy.
Without constantly utilizing energy, living matter would (click one):
It is the ability to utilize energy in appropriate ways that enables living systems to achieve the control over themselves and their surroundings which distinguishes them so clearly from non-living systems.Non-living systems have energy available to them, but they cannot utilize it appropriately either to control themselves or their surroundings. A heap of matches resting on a table can only respond to the forces and objects which act upon it. It lacks (click one):
- a. the energy available to living matter
- b. the degree of control available to living matter
- c. the entropy available to living matter
Given appropriate utilization's of energy, matches could be placed in any number of conceivable locations and positions. The mechanisms for such movements, however, are not available to the matches because they are not a living system. Only living systems can make the appropriate use of energy necessary to fight the entropy trend. Non-living systems do not possess the necessary mechanisms to use energy.
It is only through appropriate energy utilization that living matter can maintain the high degree of internal organization necessary to conduct the behaviors of matter called "life." The living system can grow, repair itself, reproduce, and organize its surroundings. If a living system did not constantly expend energy in order to maintain its high degree of organization, it could not prevent the (click one):
Which is more complex -- a person or a rock? The answer to this question is obvious. People are of course more complex than rocks. Think of all the things humans do which rocks never dream of doing-dream, for example. A rock cannot do that. Neither can it laugh, write a letter, ride a bicycle, carry in the groceries, nor do a multitude of other things that people commonly do. A rock is nonliving matter; people are living matter. The behaviors of living and nonliving matter are very different.All matter is composed of elements, and it should interest you to know that the elements which make up living matter are not particularly special. In fact, the elements that compose the chemical compounds of living matter are the same elements which make up much of nonliving matter. Carbon, oxygen, hydrogen -- these elements are important components of living matter, but they do not belong to living matter alone. They occur in rocks, water, and the air, as well. Living matter does not contain any (click one):
While elemental composition is not of great importance in distinguishing living matter from nonliving matter, there are tremendous differences in complexity between the two forms of matter. As a person is much more complex than a rock, so is living matter more complex than nonliving matter.The differences in complexity are apparent even at the molecular level. In general, the molecules which compose the chemical compounds in living matter are larger and much more complex than those in compounds which make up nonliving matter. This means that atoms in living matter are (click one):
- a. less highly organized than the atoms in nonliving matter
- b. more highly organized than the atoms in nonliving matter

Suppose you were to mix together, in exactly the right proportions,
all of the proper compounds (organic and inorganic) required to make living
matter. Would you have living matter? (Click one.)
A mixture of all the right compounds does not produce living matter. Why not? What is wrong with such a mixture? (Click one.)
- a. It does not have all of the special elements found only in living things.
- b. The compounds are more highly organized than necessary.
- c. The compounds are not organized into the complex form we recognize as life.
Study Behavioral Objective 5.
Life as we know it is an organization of molecules into discrete functional units of life -- cells. The cell is the unit which serves as a control center and site for the conduction of such dynamic processes as growth, movement, reproduction, excretion, respiration, general metabolism, etc. It is these collective processes which we characterize as life. All of these dynamic processes are conducted by the living creature, and they are also conducted in each of the creature's cells. The living cell, like the living creature, is both (click one):
Just mixing together the proper cellular components does not produce life. While such a mixture has basically the right contents, it isn't living matter. It cannot conduct the dynamic processes of life because it doesn't have sufficient organization. In living cells, molecules are very highly organized. The picture of life is one of very high levels of (click one):- a. disorder
- b. organization
- c. simplicity
| Biosphere | the collection of all living things on earth |
| Communities | aggregates of living organisms
(woodland community, desert community) |
| Species | specific classifications of living things
(Homo sapiens, Canis domesticus) |
| Organisms | individual living things |
| Cells | functional units of living things |
| Molecular Aggregates | quasi-living molecular groupings
(viruses, DNA, RNA) |
| Molecules | organizations of atoms
(carbohydrates, proteins, carbon dioxide) |
| Atoms | fundimental units of elements; common to all matter (hydrogen, oxygen, carbon) |
| Elementary Particles | basic units of matter (protons, neutrons, electrons) |
Viruses are creatures which are extremely simple in structure, commonly consisting of two types of molecules: DNA (or RNA) and proteins. Isolated from other forms of life (for example, when kept in a sealed test tube), the virus exhibits no life activities (no duplication, use of food, respiration, movement, and so forth). For all practical purposes the virus is not alive. But, if the virus is brought into contact with an appropriate cell, it immediately "takes control" of the information management portion of the cell, and the cell becomes a factory for the production of new viruses.
At the level of the molecular aggregates, there is sufficient organization for the conduction of some "lifelike" processes, but not sufficient organization for the collection of processes we term life. The level of the molecular aggregates can be considered a transitional level between living and nonliving matter.
Above the level of the molecular aggregates, matter is sufficiently organized for the conduction of dynamic life processes. Above the cellular level there is even further organization of living matter. As you read up the chart, you can see increasingly complex organizations of living units-cells into organisms, organisms into species, etc. Living matter must have a high degree of organization in order to carry on life processes.
Write a paragraph summarizing behavioral objectives 2-5.
This is the end of lesson one. Click here to go back to
the home page and lesson two ATP Structure/Cyclic Phosphorylation.
click
Created by the Multimedia Development Lab, Academic Technology Services.
Last modified October 29, 1997.