What is biological energy? How does it work? How is it synthesized? These are questions that will be answered in this unit. Biological energy is the use of a phosphate group located in an ATP molecule.
Overview:
Living organisms to maintain entrophy at the cellular level can use
only certain types of chemical energy. Energy can be stored in energy
rich molecules such as glucose monemers which make up starch. Before
a cell can use this energy it must be transferred to smaller and more energy
transfer efficient molecules-ATP. ATP are molecules which
are able to transfer energy directly to reactant molecules during chemical
reaction within a cell. Write the general function of ATP in your notes.
Three other molecules you need to learn are NADH+H, FADH2
and NADPH2. These are coenzymes, write these
coenzymes in you notes and learn. These molecules will be used to
transfer energy from large energy rich molecules during chemical reactions
which occur in one location to a second location where the energy is transferred
into ATP molecules.
In your notes draw a diagram representing the following paragraph.
In working cells ATP is used for work. As it is used for work it is
converted into ADP + P. The energy released during this reaction is the
energy cells use to perform all of thier functons. The ADP is converted
back to ATP by a process of cellular respiration and photosynthesis. It
takes energy to form ATP from ADP. The energy used during cellular
respiration comes from energy stored in energy rich molecules such as glucose.
The energy used during photosynthesis comes from light energy.
Study Behavioral Objectives 6-9. Read the pages indicated by E-3.
Cells of all kinds and types use certain molecules for the storage and release of energy. The ATP molecule is just such a special molecular structure. While many other molecules are of major importance in the release and storage of energy, ATP is the most important molecule of this type due to its widespread use in all living systems.
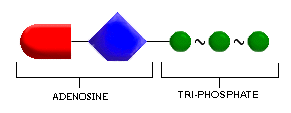
ATP is a shorthand name for the chemical compound adenosine tri-phosphate.
The adenosine part of ATP is the main body of the molecule; it is composed
of adenine (also a component of DNA) and a simple sugar, ribose. Look at
this part of ATP in the illustration.
The tri- part of the name refers to the fact that ATP has three phosphate groups linked to the main adenosine body of the molecule. The phosphate groups themselves are clusters of one phosphorus and several oxygen atoms, which are represented in shorthand form as (P) . Notice the phosphate groups in the illustration above.
The three phosphate groups of ATP are (click one):
- a. linked in a linear chain
- b. attached at random to the molecule
- c. a part of the main body of the molecule
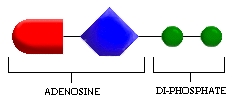
Look at the illustration and compare ADP with the illustration of ATP. What is the structural difference between ADP and ATP? (Click one.)
- a. ADP is larger.
- b. ADP has one less phosphate group than ATP.
- c. ADP has one more phosphate group than ATP.
What do you think the mono in AMP stands for?
The prefix mono means "one," and AMP has only one phosphate group on the molecule.
What do you think is the reaction for the production of AMP by a cell?
ATP, ADP, and AMP are a series of molecules which differ from each other in the number of phosphates that are attached to the base compound, adenosine. Adenosine is the basic molecule of all the molecules in the series. The cell gets adenosine either by absorbing food which contains adenosine or by making adenosine through a series of reactions. All cells have the capability of producing this important compound. Cells get their phosphate groups from the soil, in the case of plants, and from absorbed food, in the case of animals.
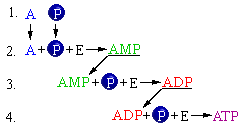
Here is the series of chemical reactions by which one ATP molecule is produced.
The final product molecule of ATP (click one):
- a. is energy rich (contains much energy)
- b. is energy poor (contains little energy)
- c. has the same amount of energy as adenosine
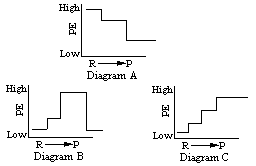
Which of the following energy diagrams represents the formation of ATP in the series of reactions given? (Click one.)
The rearrangements of atoms to produce AMP from adenosine and phosphate require energy. The AMP molecule is relatively less stable than its reactant clusters. Energy is also needed to produce ADP, and more energy is utilized to produce ATP. At each of these steps energy is required, because the cell has to invest energy when transforming stable atomic arrangements into less stable ones. The product of each succeeding chemical reaction represents a molecular arrangement (click one):
- a. lower in energy and more stable than the reactants
- b. higher in energy and more stable than the reactants
- c. higher in energy and less stable than the reactants
The final product of the series of energy storage reactions is a very high-energy molecule -- the ATP molecule. Having a supply of "unstable" molecules of ATP is of great advantage to the cell. When the cell requires energy, it can take advantage of the fact that ATP is both unstable and high in potential energy. With minimal energy input to break the phosphate bond, the cell can convert ATP back to the more stable ADP + (P) and benefit from the energy which is released by the reaction. Look at this reaction:
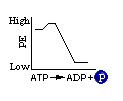
This is the energy diagram for this reaction, which is so important in providing energy for cellular use. Note the increase of energy at the beginning of the reaction. This is called activation energy and in the case of living cells to over come this "hump" an enzyme is used to allow this reaction to go to completion.
Many biology books deal with the topic of ATP energy storage and release
somewhat differently than does this book. They speak of the ATP molecule
as having "high-energy bonds" or "energy-rich bonds" which can be broken
to release
energy or established to store energy.
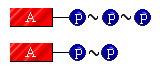
These "special" bonds are usually pictured as a "squiggle" connecting the molecular groups. Look at the illustration of the energy-rich bonds in ATP and ADP.
Biology books commonly state that ATP liberates energy from one of its high-energy bonds, releasing a phosphate group and forming ADP. The impression is thereby formed that the breaking of that bond releases energy. Since you know that energy is required to break bonds and is produced only when new bonds form, this alternative view should seem backwards to you.
You can understand this point of view if you remember that the ATP to ADP reaction must be started by the breaking of a phosphate bond. Only after this bond has been broken can the overall molecular rearrangements (which cannot be seen in these simplified reactant/product expressions) take place. It is the formation of the more stable (lower-energy) products of ADP and (P) which releases energy. The total set of changes liberates energy, but these reactions can be initiated only by the breaking of the phosphate bonds.
A high-energy bond appears special because it is the starter of a total energy-releasing chemical reaction. Without breaking a phosphate bond, the reaction cannot occur.
The two sentences below should make clear the distinction. Click on the one that correctly states the importance of the high-energy phosphate bond in the ATP to ADP reaction.
- a. Breaking the high-energy phosphate bond releases energy.
- b. Breaking the high-energy phosphate bond triggers an energy releasing reaction.
The high-energy bond model is a useful one because of its simplicity. It is much easier to picture the squiggle bond in illustrating complicated chemical reactions than it is to draw energy diagrams. The following diagrams represent the same reaction, the production of ADP from AMP.
![]()
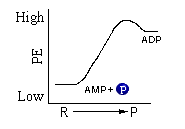
Both representations show that the ADP molecule is (higher/lower) in energy than are the reactant molecules.
The cell is able to store energy through the following chemical reactions (represented using squiggle bonds):
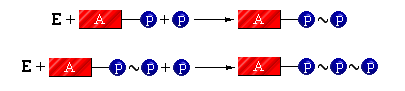
The structural representation for the reaction releasing energy to the cell during the ATP ---> ADP reaction is:
The rearrangement of atoms in the ATP to ADP reaction produces stable molecular arrangements and liberates energy for cellular use. For example, when ATP is converted to ADP and (P), a more stable set of molecular arrangements is formed. The overall reaction, initiated by the breaking of the high-energy phosphate bond, releases energy.
Which of the following statements about the ATP to ADP reaction is more accurate? (Click one.)
- a. The ATP molecule contains more energy than the product molecules.
- b. The phosphate bond of ATP contains more energy than the phosphate bond of ADP.
Both of these molecules may be reduced by various chemical reactions
(respiratory). Example: NAD
+ CH3CH2OH ---> NADH + CH3COOH
Note the NAD molecule producing a hydrogen atom.
These two reduced forms (NADH+H and FADH2) can then be transferred to the cytochrome system in a mitochondria where they will be oxidized with the energy being released converted into working ATP. Oxygen is required for this process. Study the figure in text:
ADP + P + NADH ---> NAD + ATP
ATP can be synthesized from energy rich molecules in two ways.
- Energy is directly taken from a substrate to form ATP from ADP and (P). This is called substrate phosphorylation. (single step process)
- Energy is taken from an energy-rich molecule by reducing NAD+ or FAD to NADH+H or FADH2 These molecules move to a cytochrome system in a mitochrondrion where the reduced forms NADH+H or FADH2 are oxidized to NAD+ or FAD and the accompanying energy being transferred to make ATP. This is called oxidative phosphorylation. (two step process)
This is the end of lesson two. Click here to go back to
the home page and lesson three Respiration Overview.
click
Created by the Multimedia Development Lab, Academic Technology Services.
Last modified October 29, 1997.