Read behavioral objectives 10-13, then read the pages indicated by E-4.
Cellular respiration is the chemical process (oxidation) of breaking down energy rich molecules (C6H12O6) into energy poor molecules (CO2 + H2O) with the energy being converted into biologically usable energy -- ATP. This is a slow process and requires several reactions which can be categorized into steps.
Aerobic respiration has three stages: glycolysis, the citric acid cycle (Krebs cycle), and the electron transport system. A limited amount of ATP is produced directly from specific steps in gylcolysis and the Kreb cycle (substrate phosphorylation). However, the bulk of ATP will be formed by the formation of NADH+H and FADH2 via glycolysis and the Kreb cycle which then must move to the electron transport system to be transformed into ATP using oxygen (oxidative phosphorylation). The overall equation for aerobic respiration is:
All living things require a constant input of biological energy. Although living systems get their energy from different sources and in different ways, the ultimate energy source is the same -- the sunlight energy from the sun is stored in the carbohydrates that are manufactured by green plants through the process of photosynthesis.
The carbohydrate compounds produced in photosynthesis serve as primary energy sources for most living systems. When you eat a potato, you are taking in carbohydrate molecules that have chemical energy. When you eat steak, you are taking in animal molecules which derived their chemical energy from plant molecules, which got their energy via photosynthesis. Both plant and animal cells, however, convert carbohydrate molecules into a variety of energy-rich organic compounds which can be used as sources of chemical energy.
The chemical energy in an organic compound must be released in a form usable by the living system. The process that accomplishes this task is called cellular respiration. Through cellular respiration the energy stored in complex energy-rich molecules (organic fuels) is released for use in cellular and life activities.
The cellular respiration process, like photosynthesis, is a set of complex chemical reactions which involve the breaking and forming of chemical bonds. Cellular respiration, however, involves overall energy release, whereas photosynthesis is an energy absorption process.
Cellular respiration is:
- a. a process in which complex organic matter is built from simpler matter
- b. a process in which highly organized matter is converted to simpler matter
- a. a process in which complex organic matter is built from simpler matter
- b. a process in which highly organized matter is converted to simpler matter
The burning of wood is a process which, like cellular respiration, also converts energy- rich organic compounds (cellulose, starch, and so forth) into low-energy compounds (carbon dioxide and water). When wood is burned the chemical bonds in the cellulose molecules are broken, and atoms are rearranged to produce simpler substances. Energy from the burning reaction is released in the form of light and heat as new stable compounds form.
Rapid oxidation, such as occurs when wood burns, would not be a good method for the recovery of energy by a living system because it produces energy too fast. Such a reaction would destroy the fragile organization of the living system.
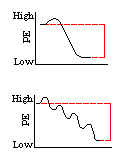
In cellular respiration, the energy of the organic molecules which store energy must be released in a highly controlled manner. The illustration to the left represents the difference between the energy-release processes of cellular respiration and wood burning. Click on the energy diagram which represents "the burning of wood."
Cellular respiration involves a step-by-step release of energy as its high-energy reactant molecules are converted to low-energy product molecules. The high-energy reactant molecules of this process are termed organic fuels. Fats, carbohydrates, proteins, and many other organic molecules high in energy content can serve as organic fuels in the cellular respiration process.
The carbohydrate molecule glucose will be used in the following chapters of this book as a simple organic fuel to illustrate the details of the process. You should remember that sugars, starches, and cellulose are all carbohydrates with the simplified formula [CH20]n, where the "n" symbolizes that CH20 is contained in the molecule a number (n) of times. Glucose is a kind of sugar, so you know that glucose is also a carbohydrate.
Glucose, like other carbohydrates, is produced in the process of photosynthesis. It is an organic fuel in which energy from the sun is incorporated as chemical energy.
A molecule of glucose (C6H1206) contains twenty-four atoms-- 6 carbon, 12 hydrogen, and 6 oxygen. Which carbohydrate formula listed is the appropriate one for glucose? (Click one.)
During the process of cellular respiration, the chemical energy in glucose is released and made available for cellular use. Several kinds of energy-releasing chemical reactions are employed to achieve this end. But, in every case, each energy-releasing chemical reaction involves the formation of product molecules which are (lower/higher) in chemical energy content than the reactant molecules.
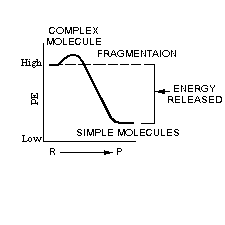
Sometimes an energy-releasing reaction involves the breaking of a molecule into simpler and more stable product-molecules.
Which of the following chemical reactions is the general reactant/product expression for the fragmentation process? (Click one.)
- a. fuel fragments + energy
- b. fuel + oxygen CO2 + H20 + energy
- c. fuel fragments + hydrogen + energy
A molecule does not always break into identical fragments but can form more than one simpler substance as fragmentation occurs. Which reactant/product expression illustrates such a process? (Click one.)
An important process for releasing energy from an organic fuel uses dehydrogenation. Sometimes the fragmentation process involves the low-energy breaking of bonds holding hydrogen atoms in the molecular arrangement of the fuel. When hydrogen is removed from an organic fuel like sugar, the process of dehydrogenation has taken place. Look at this fragmentation which illustrates the process of hydrogenation:The removal of a hydrogen atom from a glucose molecule (C6H1206) is an example of an energy-producing dehydrogenation reaction.
You now know two processes that can be used in carbohydrate metabolism to liberate energy from organic fuels. They are fragmentation and dehydrogenation.
After removal of the hydrogen atom from an organic fuel in the dehydrogenation process, the hydrogen atom itself is available to join other atoms or molecular groups. A substance which accepts the hydrogen atom is termed a hydrogen acceptor. Once of the most important hydrogen acceptors is oxygen.
If oxygen atoms are present to act as hydrogen acceptors, the reaction below can take place.
As a general rule, when a substance combines with oxygen in an oxidation reaction, the reaction will release much energy. The acceptance of hydrogen atoms by oxygen and the resultant production of water is indeed a reaction which liberates a lot of energy. If such oxidation can occur, more energy is released than would have been released if only fragmentation and dehydrogenation had occurred.
Three major energy-releasing processes used by a cell are fragmentation, dehydrogenation, and oxidation.
Cellular respiration is the process whereby energy is released from complex molecules and made available to the cell. Fragmentation and dehydrogenation reactions are often energy- yielding reactions. When sufficient amounts of oxygen are present, the cell can also utilize oxidation reactions for energy release. When an organic fuel such as sugar is completely broken down with the help of all three of these reactions, the final products are carbon dioxide and water. Any time an organic fuel is broken down to the point where just carbon dioxide and water remain, all the available energy has been extracted from the organic fuel. It is clear then, that for the maximum production of energy from a sugar molecule, the cell must have a supply of oxygen.
In the presence of oxygen, complete oxidation of carbohydrates can occur. The reactant/product expression for the complete oxidation of glucose is:
Read and study behavioral objectives 10.
Read the pages indicated by E-4
in the text and study the figures.
This is the end of lesson three. Click here to go back
to the home page and lesson four Respiration - Glycolysis.
click
Created by the Multimedia Development Lab, Academic Technology Services.
Last modified October 29, 1997.