Study behavioral objectives 14-17. Read the pages indicated in the text by E-5.
Upon completion of this "road map", draw a input/output box
in your notes indicating what enters and leaves this pathway.
Include the organic molecules and the biological energy.
Glucose, a sugar molecule, is one of several carbohydrate molecules which may result when green- plant cells conduct the process of photosynthesis. Glucose is an organic fuel, high in energy content. During cellular respiration you will see how a plant or animal cell, through a complex set of chemical reactions, begins the process of systematically and effectively extracting the chemical energy contained in the glucose molecule. The energy obtained can be stored for later use by the cell in the form of (click one):
The process through which the cell extracts energy from the glucose (or other carbohydrate) molecule is called cellular respiration. This energy extraction process can occur in either the presence or the absence of oxygen. If the process occurs anaerobically (without available oxygen), it is termed fermentation. If, on the other hand, carbohydrate metabolism occurs in a system which can make readily available a supply of oxygen, the aerobic reactions of respiration can take place.The form of cellular respiration (fermentation or aerobic respiration) depends upon the availability of oxygen.
Most living organisms engage in aerobic respiration. No complex form of life, whether plant or animal, can depend for energy upon the fermentation process. Some simpler life forms (such as certain bacteria, yeast cells, etc.) live in oxygen deficient environments. You would expect, therefore, that these cells could metabolize carbohydrates by the process of fermentation.
The initial steps of cellular respiration are conducted almost identically, irrespective of the availability of oxygen for the energy extraction process. This web page will explain the steps shared by both fermentation and respiration. In later chapters, the subsequent steps will be presented.
Study the figure indicated by E-6.
The first step in the energy extraction process is the phosphorylation of the glucose molecule. In this chemical reaction a phosphate group displaces a hydrogen atom from the glucose molecule, and glucose phosphate is the product. Look at the reactant/product expression for the reaction. Phosphorylation is where ATP is used to add or remove energy from an organic molecule.
Step 1: ATP + glucose + phosphate ---> glucose phosphate + ADP
(This is the first step of your road map - energy is being used and not released during this step) .
The process by which phosphate is added to a molecule is usual described as phosphorylation. It should be of interest to you that this process is an energy absorbing process; that is, energy is needed to drive the chemical reaction. Why is this point important?
Answer: The purpose of cellular respiration is to release energy to the cell, not to use it up.
The first step in a process geared toward the extraction of energy from the glucose molecule involves an investment of energy! This is called activation energy. Write the definition of this term in your notes. This is much like a football game (in which the idea is to move toward the opponents' goal) starting with the quarterback:
- a. refusing to carry the ball
- b. carrying the ball forward toward the goal
- c. stepping backwards away from the goal to throw a pass
The chemical reaction for Step 1 holds the key to determining the source of energy for the, phosphorylation reaction. If you look at Step 1 carefully, you will notice that the glucose molecule must provided with both phosphate and energy for the reaction to proceed. The cell easily obtains both needed reactants by (click one):
- a. absorbing them from the environment
- b. activating an ATP to ADP reaction
- c. creating them from raw materials
After the glucose molecule has been phosphorylated, it undergoes a rearrangement of atoms and takes on a slightly different shape. The new molecular arrangement is that of fructose phosphate.
Step 2: glucose phosphate ---> fructose phosphate
In energy terms, the molecular rearrangement in Step 2 is not consequential, but you should expect that since the new arrangement is slightly more stable, just a very small amount of energy would be (released/absorbed).
In the third step of the carbohydrate metabolism process, fructose phosphate reacts with another phosphate group.
Step 3: ATP + fructose phosphate + (P) ---> fructose diphosphate + H
(During this step another phosphate is added to the organic molecule. How many ATP does it take to activate glycolysis? 2 ATP per glucose molecule)
Again, hydrogen is displaced by a phosphate molecule. Which term bests suits this reaction? (Click one.)
- a. oxidation
- b. phosphorylation
- c. fragmentation
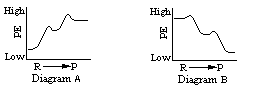
As with Step 1, the Step 3 chemical reaction is powered by the conversion of an ATP molecule to ADP. Which of these energy diagrams represents the energy changes that have occurred thus far in the process of carbohydrate metabolism? (Click one.)
In the forth step, the fructose diphosphate is split in half, forming two PGAL molecules:
(In your road map indicate that the single six carbon fructose molecule and been split into two three carbon molecules of PGAL.
Later you will learn that PGAL is also used when light energy is used to synthesize energy rich molecules.
In what other process does PGAL play an important role?
- a. respiration
- b. photosynthesis
- c. combustion
In photosynthesis the PGAL is:
In respiration the PGAL is: During the first four reactions in the breakdown of glucose, there is no release of energy. In fact, since the two phosphorylations are accomplished by the conversion of 2 ATP molecules to 2 ADP molecules, there is: This represent activation energy being added to the organic moleucle. In these first four reactions, glucose is changed to PGAL:
2 ATP + C6H12O6
---> 2C3H5O3
- 2(P) + 2 ADP
Check this summary of reactions with the steps included in your road map.
Let's balance this equation:
On the input side there are six carbon atoms. There are how many carbon atoms in the two molecules of PGAL on the output side of the equation?
Answer: Six in the two molecules.
There are 12 hydrogen atoms in the original glucose, but in the two PGAL molecules there are only two hydrogen atoms. Each time a phosphate group was added to the molecule, one hydrogen atom was released.
So, in two phosphorylations there would be a release of H hydrogen atoms. Now we can balance the equation:
This equation shows that the net change in the breakdown of glucose, through the first four reactions, is the loss of hydrogen atoms.
Removal of phosphate "P" from ATP is accompanied by a large release of energy; the liberation of phosphate from an inorganic acid is not accompanied by a large release of energy. In the fifth reaction below , phosphate from phosphoric acid displaces a hydrogen atom from each PGAL molecule, and two molecules of diphosphoglyceric acid are produced.
Step 5:
2 NAD + 2PGAL + 2 (iP) ---> 2 diphosphoglyceric acid
+ 2 NADH
Note: Two hydrogens are pulled from PGAl by NAD to FORM NADH.
These NADH molecules will be used in the third set of reactions "CETS" to form 2 ATP per NADH (write in your notes).
The fact that no energy is stated on either side of the equation must mean that (click one):
- a. no energy is liberated during the reaction
- b. no energy is used during the reaction
- c. any energy changes are so small that they are of no consequence
Step 6:
2 ADP + 2 diphosphoglyceric acid ---> 2 phosphoglyceric
acid + 2ATP
Note: The diphospho- changed to a phospho-
The phosphates and energy of this reaction are available for entry into energy storage reactions so that the energy released in Step 6 will not be lost by the cell. This is the reactant/product expression for this energy storage reaction:
Two molecules of ATP can be produced from the two phosphate groups liberated in Step 6. The overall reactions in Step 6 yield two ATP's for energy storage. In our football analogy the team has started to move forward toward the opponents' goal line. In energy terms, the metabolism process is at last (click one):
- a. making energy available rather than using it
- b. absorbing energy rather than releasing it
- c. producing valuable compounds for future reactions
This clearly shows how these two reactions are linked to establish an energy transfer from one set of reactions to a second set of reactions.
In the seventh step, phosphoglyceric acid is converted to pyruvic acid.
Step 7:
2 ADP + 2phosphoglyceric acid ---> 2 pyruvic acid + 2 ATP
If you examine Step 7 carefully, you will note that, as in the previous step, two phosphate groups and energy are produced. These products can be used by the cell to produce (click one):
Step 7, like the last step in the metabolism process, is linked to an ADP/ATP reaction so as to store, for future cellular use, the energy produced in the step. This is Step 7 showing the energy storage process along with the energy producing step.Here is a summary of the seven steps in glycolysis metabolism:
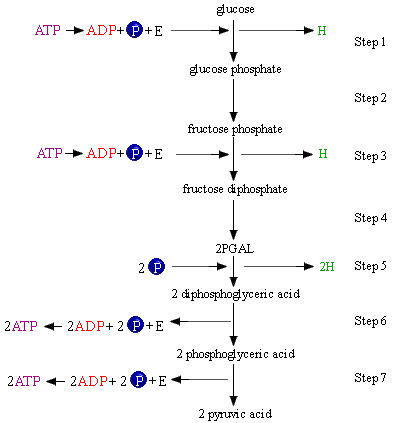
Note: Step 5 NAD is reduced to NADH
During glycolysis of one glucose molecule both energy consuming and energy producing chemical reactions have been used . Two ATP's were converted to ADP to fuel initial reactions, while four ADP's were later converted to ATP's.
How many ATP molecules of stored energy have been made available to the cell by the first seven steps in the process of carbohydrate metabolism?
Answer: Two.
If we look at the initial reactant molecule (glucose) and of the final products of this set of seven reactions, we can write reactant/product expression for the overall set of reactions. It is:
Carbohydrate metabolism has extracted from the original glucose fuel enough energy to build two ATP molecules. One of the products, pyruvic acid, is a complex molecule. More energy could be gained by the cell if it could metabolize this molecule. The other product, NADH, also provides energy (equilivent to 2 ATP) when they are passed through a cytochrome electron transport system. i
input/output box
(write in your notes and learn)
Organic Molecules - Input: glucose
Output: 2 pyruvic acid molecules
Energy Output - ATP ( 2 used during activation
and 4 synthesized = net of 2)
NADH (2 formed which may be converted into 2 ATP per NADH
thus a total of four ATP may be formed by these coenzymes)
Glycolysis produces a net of 2 ATP directly and 4 through NADH, a total of 6 ATP per glucose.
Substrate phosphorylation refers to the ATP made directly.
Oxidative phosphorylation refers to the ATP synthesized through the
NADH
There are two routes by which the cell can continue the energy extraction process beyond the breakdown to pyruvic acid. Which route the cell takes depends on the amount of oxygen available to the cell.
You will recall that if plenty of oxygen is available to the
cell, the carbohydrate metabolism becomes an aerobic respiration
process. If oxygen is not available to the cell, the process
is one of anaerobic respiration or fermentation. While both processes
share the initial stages already described on this web page, their subsequent
reactions differ greatly. The following web pages describes anaerobic respiration
or fermentation.
This is the end of lesson four. Click here to go back
to the home page and lesson five Respiration-Anaerobic Respiration.
click
Created by the Multimedia Development Lab, Academic Technology Services.
Last modified October 29, 1997.