During glycolysis and the Kreb cycle reduced NADH+H and FADH2 are formed. These reduced forms of coenzymes will use their stored energy to make ATP (oxidative phosphorylation). This system will only work if oxygen is present. Study behavioral objectives 23-24 and read the pages indicated by E-12.
The glucose molecule is now completely oxidized. Some of its energy has been used to produce ATP from ADP and phosphate. Most of its energy, however, remains in the electrons removed from the carbon atoms and passed to the electron carriers NAD+ and FAD. These electrons -- from glycolysis, the oxidation of pyruvic acid, and the Krebs cycle -- are still at a high energy level.
In terminal electron transport, which is the final stage of respiration, these high-energy-level electrons are passed step-by-step to the low energy level of oxygen. This stepwise passage is made possible by the electron transport chain, a series of electron carriers, each of which holds the electrons at a slightly lower energy level. Among the principal components of the electron transport chain are molecules known as cytochromes. Although the structures of the individual cytochromes are similar, they differ enough to enable them to hold electrons at different energy levels.
As the electrons move through the electron transport chain, dropping to lower energy levels, energy is released. This energy is harnessed by the mitochondrion to power the synthesis of ATP from ADP in a process known as oxidative phosphorylation. Quantitative measurements show that for every two electrons that pass from NADH to oxygen, three molecules of ATP are formed from ADP and phosphate. For every two electrons that pass from FADH2, which holds them at a slightly lower energy level than NADH, two molecules of ATP are formed.
With the synthesis of ATP in oxidative phosphorylation, the process
that began with the glucose molecule is complete. However, before we sum
up the total energy harvest, let us look more closely at how ATP is synthesized
in conjunction with terminal electron transport.
What is the fate of the hydrogen atoms of NADH and FADH generated by glycolysis and the Krebs Cycle? Experiments show that they are not immediately oxidized to water, but rather they enter a complex set of chemical reactions Electron Transport Chain or CETS, which release their energy in bits and pieces. Direct oxidation of hydrogen would release the energy to the cell much more quickly than does the lengthy series of reactions used in oxidative respiration. Why would the more time-consuming process be favored by the cell?
Answer: The cell cannot handle the rapid release of a lot of energy.
Rather than traveling directly to oxygen atoms in this reaction,
H + H + O---> H2O + E, the hydrogen atoms travel a
pathway called a respiratory chain. The respiratory chain of reactions
leads them through a number of different acceptors to their eventual acceptance
by oxygen atoms. (In reality, the hydrogen atom splits into an electron
[e-] and a proton [p+]
which travel separate pathways to meet an oxygen atom. We shall not complicate
matters further by presenting both pathways, but rather shall speak of
the two as if they were combined and traveling the respiratory chain together.)
Study figure in text showing the electron transport system in the
mitochondria.
NADH + H and FADH2 serve as the first molecule entering the CETS. The equation below represents the formation of NADH and FAD2 as it occurred in the Krebs and glycolysis.
NAD + 3-CH5 --------> NADH + H + 3 C-H3
As the reactant/product expression indicates, this first stage (click one):
The product of the first reaction, NADH, now enters into a reaction with a second molecule, FAD.NAD-H is not as stable a molecule as is the combination of FAD and hydrogen. When the FAD molecule comes into contact with NAD-H, it "steals" the hydrogen from the NAD and forms the more stable FAD-H. Since this step produces a more stable configuration for the atoms, it is a reaction which (click one):
- a. uses energy from the environment
- b. releases energy to the cell
- c. is of no consequence in energy terms
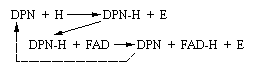 Look
at this illustration of the two reactions already described. NAD may be
substituted for DPN in these figures.
Look
at this illustration of the two reactions already described. NAD may be
substituted for DPN in these figures.
When NAD is rid of its hydrogen in the second reaction, what is it free to do?
Answer: It is free to pick up a new hydrogen and thus start Reaction 1 again.
In the third reaction of the respiratory chain, FAD-H reacts with
the molecule cytochrome, which repeats the process of attracting
the hydrogen away from another molecule and effectively robs FAD-H.
Since the cytochrome-H is a more stable molecule than FAD-H, the reaction releases a third bundle of energy to the cell.
Cytochromes will repeat the processes above with other cytochromes until you get to the end of the respiratory chain.
Finally, the cell brings cytochrome-H into contact with oxygen. (Two hydrogen atoms are required to produce one water molecule, so two cytochrome molecules react with one oxygen atom in this reaction.)
As you can see, this too is an energy-liberating reaction. The set of reactions will stop at this point because water is a very stable compound, and "stealing" the hydrogen away from the oxygen in water uses energy rather than releases it.
To help you get a better feel for the series of reactions in the respiratory chain, let's look at all of them together: NAD = DPN
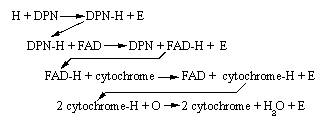 You
can see that (with the exception of oxygen) each hydrogen acceptor which
snatches a hydrogen atom is regenerated in a subsequent reaction as its
hydrogen "prize" is snatched away. Essentially, the real reactants
and products of the respiratory chain of hydrogen transfer can be summarized
in the reactant/product expression:
You
can see that (with the exception of oxygen) each hydrogen acceptor which
snatches a hydrogen atom is regenerated in a subsequent reaction as its
hydrogen "prize" is snatched away. Essentially, the real reactants
and products of the respiratory chain of hydrogen transfer can be summarized
in the reactant/product expression:
The important concept that would be overlooked when this summary reaction is written to represent the process would be the fact that the energy is liberated in a series of small steps would be overlooked.
Each transfer of the hydrogen from one acceptor to another involves the regeneration of the reactant molecule of the previous step. NAD is regenerated to be used again, as is FAD and cytochrome. The compounds NAD, FAD, and cytochrome are called intermediary compounds. They take place in intermediary reactions, but they themselves are unchanged in the process and they can be used over and over again. They simply pass hydrogen molecules down the respiratory chain to oxygen (which is used up in the process).
The energy liberated as a hydrogen atom moves from hydrogen acceptor to hydrogen acceptor is best illustrated by which of these energy diagrams? (Click one.)
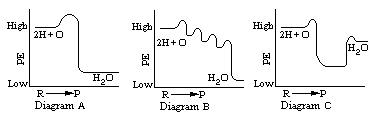
Answer: Diagrams A and B can both be used to represent the same
overall chemical reaction, the oxidation of hydrogen. Diagram A shows the
sudden release of energy, while Diagram B shows the more gradual release
of energy in a series of steps. Diagram A may represent energy release
in physical systems (such as the combustion of hydrogen), but Diagram B
represents the way in which the energy is released in biological systems
when hydrogen is oxidized through the respiratory chain.
What is the relationship between the electrons transport chain and
the synthesis of ATP?
Two steps during chemiosmotic coupling:
1. The electron chain runs a proton pump (described below). This pump across membranes in the mitochondrion will cause an osmotic gradient to form across membranes of the mitochondrion. This osmotic pump will allow the enzyme ATP ase (synthatase) to be activated.
2. ATP ase (synthetase) will phosphorylate ADP as follows:
ADP
+ Pi -------->
ATP + HOH
Study the figures on the pages indicated by E-13. The mechanism of oxidative phosphorlylation was, until the early 1960s, one of the most baffling puzzles in all of cell biology. As a result of the insight and experimental creativity of the British biochemist Peter Mitchell (1920-1992) -- and the subsequent work of many other investigators -- much of the puzzle has now been solved. Oxidative phosphorylation depends on a gradient of proteins (H+ ions) across the mitochondrial membrane and the subsequent use of the potential energy stored in that gradient to form ATP from ADP and phosphate.
The components of the electron transport chain are arranged sequentially in the inner membrane of the mitochondrion. Most of the electron carriers are tightly associated with proteins embedded in the membrane, forming three distant complexes. According to current evidence, these complexes are locked into place in the membrane. Within each complex, the electron carriers are held in the proper positions in relation to one another.
The protein complexes also have another crucial function: they are proton pumps. As the electrons drop to lower energy levels during their transit through the electron transport chain, the released energy is used by the protein complexes to pump protons from the mitochondrial matrix into the intermembrane space. It is thought that for each pair of electrons moving down the electron transport chain from NADH to oxygen, 10 protons are pumped out of the matrix.
The inner membrane of the mitochondrion is, as we noted earlier, impermeable to protons. Thus, the protons that are pumped into the intermembrane space cannot easily move back across the membrane into the matrix. The result is a concentration gradient of protons across the inner membrane of the mitochondrion, with a much higher concentration of protons in the intermembrane space than in the matrix.
Like a boulder at the top of a hill or water at the top of a falls, the difference in the concentration of protons between the intermembrane space and the matrix represents potential energy. This potential energy results not only from the actual concentration difference (more hydrogen ions outside the matrix than inside) but also from the difference in electric charge (more + charges outside than inside). The potential energy is thus in the form of an electrochemical gradient. It is available to power any process that provides a channel allowing the protons to flow down the gradient back into the matrix.
Such a channel is provided by a large enzyme complex known as ATP synthatase. This enzyme complex, which is embedded in the inner membrane of the mitochondrion, has binding sites for ATP and ADP. It also has an inner channel, or pore, through which protons can pass. When protons flow through this channel, moving down the electrochemical gradient from the intermembrane space back into the matrix, the energy released powers the synthesis of ATP from ADP and phosphate.
This mechanism of ATP synthesis is known as chemiosmotic coupling. The term "chemiosmotic," which was coined by Peter Mitchell, reflects the fact that the production of ATP in oxidative phosphorylation includes both chemical processes and transport processes across a selectively permeable membrane. As we have seen, two distinct events take place in chemiosmotic coupling: (1) a proton gradient is established across the inner membrane of the mitochondrion, and (2) potential energy stored in the gradient is used to generate ATP from ADP and phosphate. Study the figure indicated by E-14.
Chemiosmotic power also has other uses in living systems. For example, it provides the power that drives the rotation of bacterial flagella. In photosynthetic cells, as we shall see in the next chapter, it is involved in the formation of ATP using energy supplied to electrons by the sun. And, it can be used to power other transport processes. In the mitochondrion, for example, the energy stored in the proton gradient is also used to carry other substances through the inner membrane. Both phosphate and pyruvic acid are carried into the matrix by membrane proteins that simultaneously transport protons down the gradient.
We noted earlier that "about" 36 molecules of ATP are formed for each molecule of glucose oxidized to carbon dioxide and water. The exact amount of ATP formed depends on how the cell apportions the energy made available by the proton gradient. When more of this energy is used in other transport processes, less of it is available for ATP synthesis. The needs of the cell vary according to the circumstances, and so does the amount of ATP synthesized.
SUMMARY of CETS
CETS takes energy from reduced forms of NADH and FADH2 formed during glycolysis and Krebs and converts it into ATP molecules. During this process hydrogen ions are formed and are neutralized by molecular oxygen to form water.
input/output box
(write in your notes and learn)
Organic Molecules - Input: NADH, FADH2,
O2
Output: NAD, FAD (recycled) water (HOH)
Energy Output -
ATP ( 3 ATP per NADH which enters and 2 ATP per FADH2
which enters)
This process forms ATP by oxidizing NADH and FADH2
(oxidative phosphorylation)
Substrate phosphorylation refers to the ATP made directly from a substrate.
Oxidative phosphorylation refers to the ATP synthesized through the
NADH
or FADH2 utilizing the cytochrome electrons
system (CETS).
This is the end of lesson seven. Click here to go back
to the home page and lesson eight: Mitochondria.
click
Created by the Multimedia Development Lab, Academic Technology Services.
Last modified October 29, 1997.