
Macromolecules which make up living organisms can be classified into four main classes: carbohydrates, lipids, protein, and nucleic acids. This lesson will teach you to learn to recognize the molecular and structural formulas and the characteristic properties for these four groups.
Carbohydrates: Sugars, Starch and Cellulose
Read the pages in the text indicated by A-10
and examine behavioral
objectives 25, 27, and 28.
Carbohydrates are macromolecules which consists of the elements of carbon, hydrogen, and oxygen in an approximate ratio of one C to two H to one O. CH2O (example glucose C6 H12 O6 )
Carbohydrates include monosaccharides and disaccharide (sugars) and polysaccharides (starches, glycogen, and cellulose). Examine the structural formulas for these molecules found in the readings - A-10.
Monosaccharides (molecules consisting of a single sugar monomers)
Glucose C6H12 O6 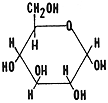
Fructose C6H12O6 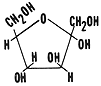
Note: In the structures above, a carbon atom (not indicated) is located
at each corner in the rings.
Glucose is the most common type of monosaccharide. Green plants produce this sugar during the process photosynthesis. This process will be investigated in miniunit Epsilon. All cells use glucose as a source of energy. Your blood sugar consists of glucose. You use this sugar as an energy source and also as a source of carbon skeletons to form other organic molecules. This is the only sugar which can be transported by your blood. All other sugars and carbohydrates must be converted into glucose before they can enter the blood stream and be transported throughout the body.
Fructose is a monosaccharide sugar found in corn syrup and fruits. When you eat corn syrup enzymes in your digestive system must first convert fructose into glucose before it can be absorbed into you bloodstream. Honey contains fructose.
1. Why is healthy for you to use honey (contains fructose) in place of sucrose as a sweetener?
Press here to check your answer.
Examine the structural formulas for glucose and fructose. Learn to
recognize the abbreviated ring structure of glucose and fructose. Note
that these two sugars have the same molecular structure and different
structural formulas.
glucose  fructose
fructose
2. What term is used to designate molecules with the same structural formulas but different molecular formulas?
Press here to check your answer.
Write in your notes the molecular and structural formula for both of
these monosaccharides.
It is easy to distinguish between these two monosaccharides. Glucose is a six sided ring and fructose is a five sided ring.
Disaccharides (molecules consisting of two sugar monomers)
Maltose (C12 H22 O11) (see molecule on the left)
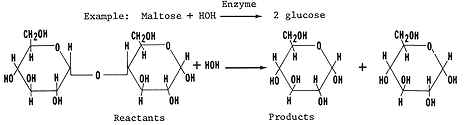
Sucrose( C12H22O11) (see molecule on the right)
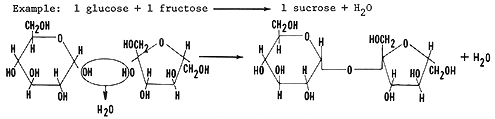
Sucrose is common table sugar. It consists of one glucose monomer covalently bonded to one fructose monomer by the condensation synthesis reactions explained above forming glycosidic linkages. Locate the glycosidic bond (C-O-C) between the two monomers.
Let us review condensation synthesis: During the process of condensation synthesis one water molecule between each monomer will be removed. Each monomer contributes part of the water molecule that is released; one monomer loses a hydroxyl group (OH), and the other loses a hydrogen (H). Both monomers, having each lost a covalent partner, now bond covalently with each other. Study diagram below. Trace the events described above using the figure below.

Glycosidic linkages means that there is the following arrangement of elements:
C-O-C Find this arrangement in the two molecules above.
Maltose is the sugar found in cereals (malt). It consists of two glucose monomers covalently bonded by the condensation synthesis reactions forming glycosidic linkages. See above to review.
Examine the structural formulas for maltose and sucrose. Learn the abbreviated ring structure of maltose and sucrose. Maltose has two six sided sugars covalently bonded together and fructose has a six sided sugar and a five sided sugar covalently bonded together. Note that these two sugars have the same molecular formula but a different structural formula.
3. Sucrose and maltose are:
- a) isotopes
- b) isomers
- c) ionic molecules
- d) polysaccharides
- e) all of these
Study the figure showing the hydrolysis of sucrose or maltose. As discussed
earlier water is removed from the disaccharide when it is hydrolyzed.

Let us review hydrolysis: During the process of hydrolysis large molecules are broken into its individuals monomers. This process is the reverse of condensation synthesis. Bonds between monomers are broken by the addition of water molecules, a hydrogen from water bonds to one monomer, and a hydroxyl bonds to the adjacent monomer. Study the diagram above. Trace the events described above using the above.
At this point let us learn more about chemical equations. Chemical equations are a shorthand way of writing chemical reactions. The compounds listed behind the arrow are the reactants (the molecules which are going to be used during the reaction). The compounds listed in front of the arrow are the products (the molecules which are going to be formed). All equations must be balanced, that is the number of atoms for each element of the reactants must equal the number of atoms for each element of the products.
4. What products would be formed during the hydrolysis of maltose?
Press here to check your answer.
5. This morning you placed sucrose on your cereal. Presently this sucrose
is being digested (hydrolyzed) in your large intestine. What products are
being formed in your large intestine?
Press here to check your answer.
6. Write a balanced equation showing the synthesis of sucrose.
Press here to check your answer.
Examine the molecular formulas for these two disaccharides. Note that
even though they are made up of two monosaccharides with the the
molecular formula of C12H22O11
, their molecular formula is not C12 H24O12.
Explain the reason for these differences and why it was stated earlier
that carbohydrates have an approximate ratio of one C to two H to one O.
7. Press here to check your answer.
Write in your notes the molecular and structural formula for both of
these disaccharides.
Polysaccharides (giant chains of monosaccharides
connected by glucosidic linkages)
In most cases glucose is the monomer; however some biological molecules
will have other sugar monomers as part of the polymer chain.
Example of polysaccharides are: starch, glycogen, cellulose.
Starch is large family of giant molecules of similar structure. All starches consist of sugar monomers (usually glucose) which are covalently bonded by alpha 1-4 linkages. See figure below. Example of starch are corn and potato starch. Bread, chips, cereals are mostly made up of starch. Most plant starches are straight chains of glucose monomers with little branching.
Glycogen is similar to starch found in plants. Glycogen is more highly branched and found in animal tissues. Your liver contains glycogen which can be hydrolyzed into glucose when the blood sugar gets low on sugar and can be synthesized into glycogen when there is too much glucose in the blood.
Cellulose consists of glucose subunits similar to starch. Cellulose, however has beta 1-4 linkages between the sugar monomers. This beta 1-4 linkages causes every other monomer in a cellulose polymer to be inverted. Examine the structural formula of cellulose in the text. These beta linkages give cellulose (polysaccharide) completely different properties from that of starch (also a polysaccharide). Paper and wood are made of cellulose. The cell walls of plant cells consist of cellulose. Even though starch and cellulose are made up the same glucose monomers your digestive system can not digest cellulose into glucose but it can digest starch into glucose. Termites can digest cellulose because they have protozoa and bacteria in their stomach which can hydrolyze cellulose into glucose. Herbivores such as deer and cattle have an extra pouch in their digestive system which contain bacteria and protozoans that can digest cellulose into glucose.
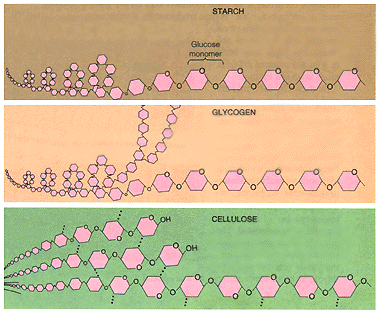
Note: The top figure represents starch. The glucose polymers are in a chain fashion (1-4 linkages) with no branching. The "1" carbon is the carbon in the ring below the oxygen located in the ring. The next carbon (clockwise) is "2" followed by "3", "4", and finally "5".

Note: The middle figure represents glycogen. The glucose polymers are in a chain fashion (1-4 linkages) with branching between "1" and "5" linkages. The "1" carbon is the carbon in the ring below the oxygen located in the ring. The next carbon (clockwise) is "2" followed by "3", "4", and finally "5".

Note: The bottom figure represents cellulose molecule. Celluose is
similar to starch and glycogen in that the monomers are glucose. However,
the 1- 4 glycosidic linkages are of beta form and not alpha form. This
causes every other monomer to be inverted.
Examine the structural formulas located in the text (A-11) for these three polysaccharides. In your notes draw a structural formula of these three polysaccharides.
All three of these polysaccharides are formed by the condensation synthesis of many glucose monomers. Let us review condensation synthesis: During the process of condensation synthesis one water molecule between each monomer will be removed. Each monomer contributes part of the water molecule that is released; one monomer loses a hydroxyl group (OH), and the other loses a hydrogen (H). Both monomers, having each lost a covalent partner, now bond covalently with each other. Study diagram below.
All three of the polysaccharides can be hydrolyzed (digested) into their individual monomers.
Let us review hydrolysis: During the process of hydrolysis large molecules are broken into its individuals monomers. This process is the reverse of condensation synthesis. Bonds between monomers are broken by the addition of water molecules, a hydrogen from water bonds to one monomer, and a hydroxyl bonds to the adjacent monomer. Study the diagram below.
8. Hydrolysis of cellulose will produce:
- a) sucrose
- b) many glucose monomers
- c) one glucose monomer
- d) fructose monomers
9. The bread you had for breakfast this morning was made up of starch.
It is presently in your stomach being digested (hydrolyzed into which compound?
Press here to check your answer.
Lipids: Fats, Oils, Steroids
Read the pages in the text indicated by A-10
and study the figures concerning lipids. Lipids are macromolecules
which contain the elements carbon, hydrogen and oxygen similar to the carbohydrates;
however, the ratio of C to O is very large. (180 C to 6 O). Lipids are
compounds which are not very soluble in water. However lipids are soluble
in organic solvents such as acetone, alcohol or oils. Molecules which are
not very soluble in water are called nonpolar molecules and molecules
which are soluble in water are polar molecules.
Learn the following solubility principle: Nonpolar molecules such as lipids and acetone will dissolve in one another; polar molecules such as water and sucrose will dissolve in one another; polar and nonpolar molecules will not dissolve in one another.
10. Cooking oil is ______ (polar or nonpolar)
Press here to check your answer.
Lipids can be divided into three groups: triglycerides (animal
fat and plant oil), phospholipids, and steroids (fused ring
structures).
Triglycerides contain one molecule of glycerol which covalently bonds with three fatty acids. These are also called fats or oils. The monomers are not hooked in a chain like fashion and therefore are not polymers. There are two different types of monomers - glycerol and a fatty acids.
Glycerol is a three carbon chain with each carbon containing an alcohol group.
Fatty acids are a long chains of carbon atoms (6-28) with an acid group (carboxylic acid functional group) on one end.
The fat or oil (triglyceride) is formed when fatty acid molecules covalently bind to each of the three alcohol groups. See below. This process is a condensation synthesis reaction as demonstrated before. Let us review condensation synthesis: During the process of condensation synthesis one water molecule between each monomer will be removed. Each monomer contributes part of the water molecule that is released; one monomer loses a hydroxyl group (OH), and the other loses a hydrogen (H). Both monomers, having each lost a covalent partner, now bond covalently with each other. This time the OH is removed from the alcohol of glycerol and the H is removed from the -OH of the acid group located on the fatty acid. Trace the events described above using the figure below.
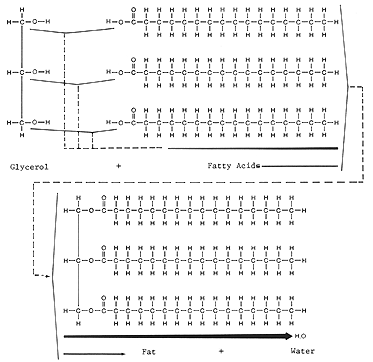
In your notes draw formulas showing the synthesis of a triglyceride from three fatty acids containing eight carbon atoms with one glycerol molecule.
Triglycerides can also undergo hydrolysis. Let us review hydrolysis: During the process of hydrolysis large molecules are broken into its individuals monomers. This process is the reverse of condensation synthesis. Bonds between monomers are broken by the addition of water molecules, a hydrogen from water bonds to one monomer, and a hydroxyl bonds to the adjacent monomer. Study the diagram below and identify the glycerol and fatty acid molecules.
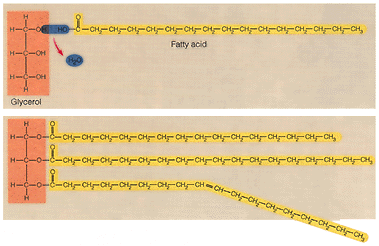
11. How many water molecules are formed during the synthesis of a triglyceride?
Press here to check your answer.
12. This morning you had butter on your toast. Butter is a triglyceride.
What are the products of triglyceride digestion (hydrolysis) which is occurring
in your large intestine?
Press here to check your answer.
Phospholipids are similar to the triglycerides except one of
the fatty acid chains is replaced by a phosphate containing group. This
phosphate containing group is very soluble in water. This in turn forms
a single molecule which has one part very water soluble (polar) and another
part which is not soluble in water. The phosphate functional group will
be hydrophilic, attracting polar water molecules. The two fatty acids are
hydrophobic and will not mix in the water. Examine and place in your notes
figures from your reading showing phospholipid structure.
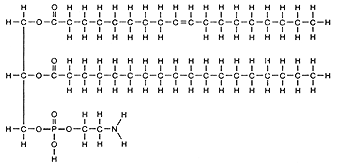
Note: Locate the hydrophilic phospholipid part of the fat molecules above.
13. What would happen if you placed this molecule in water?
Press here to check your answer.
In biological membrane which you will study in miniunit Beta phospholipids
line up in such a way that the nonpolar, hydrophobic tails pack tightly
together to form the interior of the membrane, and the phosphate containing
"heads" face outward (some on either side, where they interact with water.
The phospholipid forms a bilayer, a sheet two molecules thick. Only nonpolar
molecules will be allowed to pass through the lipid portion of this barrier.
Polar molecules will have to pass through by using special protein molecules
or pores.
14. Are phospholipids and triglycerides polymers?
Press here to check your answer.
Steroids are nonpolar organic compounds with multiple rings
which share carbons. These rings are not connected by the condensation
synthesis reactions discussed above. Example of steroids are testosterone,
estrogen, cortisone, vitamins and cholesterol.
15. Can steroids be broken down by hydrolysis?
Press here to check your answer.
The two other biological molecules you will need to learn are protein
and nucleic acids. These will be found on the next home page. Answer the
following question correctly to go to the next page.
16. Both lipids and carbohydrates contain the elements C,H, and O.
For information on how to use this page, go to How
to Use This Site.
Created by the Center for Learning Technologies, Academic Technology Services.
Last modified October 22, 1997.