
unit membrane 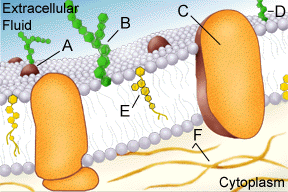
Molecules must be able to move into or out of a cell across its
membrane. In this section you will
learn how this is accomplished. Focus on the following principles as
you learn the various ways
that molecules are transported across the membrane.
1. Type of molecules ( water, small organic
molecules, small inorganic molecules) moving across
the membrane.
2. Passive process (does not require
biological energy) or active process (does require
biological energy
- ATP) moving the molecules.
3. Accomplished by special carrier protein molecules in the membrane.
Let's begin this study by writing some definitions in your notes.
Kinetic energy- energy of motion - all matter
contains kinetic energy - increase
temperature, allows matter to absorb energy and move faster - decrease
temperature allows matter to lose energy and moves slower - 0 degree Celsius
(-273 degree C) no kinetic energy - ice is water molecules (solid state)
with less
kinetic energy, when heated ice water molecules move faster and turns into
liquid,
when heated liquid water molecules move faster and turns into gas
Diffusion- molecules move from an area
of high concentration to an area of low
concentration - concentration is the number of molecules per volume
(10% = 10
molecules per 100) and amount is the number of molecules (10)
All molecules possessing kinetic energy can diffuse. Gas molecules can
diffuse
through other gas molecules, liquids and solids. Molecules in the liquid
state can
diffuse through other liquid molecules, gas molecules and solid molecules.
Equilibrium- that point where there is no
net movement of molecules - net movement is
where there is more molecules moving in one direction compared to the opposite
direction - when molecules move in both directions at the same rate there
is no
net movement.
Gradient- concentration differences
between two regions of space - sugar placed in a cup
of water initially has a high concentration of sugar around the undissolved
sugar at
bottom of cup compared to the region at the surface of the water
Diffusion - Passive Transport
Diffusion -Read the pages in the text indicated by B-19
and study behavioral
objective 18. Study the figure showing diffusion of a
dye in water. Note - No membrane is involved. Dye molecules are moving
from a region of high concentration (at dye particle) to a region of low
concentration (water space away from particle). Keep in mind that water
molecules are also diffusing; however, they are diffusing from the water
space to the area around the dye particle. This will continue until equilibrium
is reached (dye is completely dissolved in the water). At equilibrium
all of the liquid space in the beaker will have the same concentration
of dye molecules; however, the dye and water molecules will continue
to move (kinetic energy).
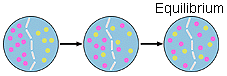
In the figure above the pink dots on the left are diffusing to
the right until the concentration of pink and yellow dots are the same.
The pink dots are diffusing to the right and the yellow dots are diffusing
to the left. The dots are still moving at equilibrium but at the
same rate.
A A A
A
___________
| p p
| A
| p p p
| A
| p p p
|A
| p p p
|
| p
p | A
|___________|
In your notes write a description explaining the diffusion
of perfume molecules from a bottle placed in the front of the lecture
room with air molecules of the room after the lid is removed. See diagram
above. (The "p" represent perfume molecules in bottle and "A" represent
air molecules outside of the bottle). When the bottle is opened the perfume
molecules will move out of the bottle due to kinetic energy and the air
molecules will move into the bottle due to kinetic energy. Some perfume
molecules which have left the bottle will move back in and some
of the air molecules which moved into the bottle will move back out (random
movement). Since there will be more perfume molecules moving out
of the bottle than into the bottle, the net movement of perfume
molecules will be out of the bottle. Using the same explanation
the net movement of air molecules will be into the bottle.
Both of the gaseous molecules are moving from a high concentration to a
low concentration. Will diffusion eventually come to a stop?
Yes, at equilibrium. When there is no net movement of either the
perfume or air molecules. (Note- molecules will always be moving in and
out.) In this case when equilibrium in reached, the number
of perfume molecules leaving the bottle is equal to the number of perfume
molecules entering the bottle.)
In your description include concentration gradient, the initial
direction of movement of the perfume molecules and air molecules, the movement
of these molecule at equilibrium, the concentration of perfume molecules
initially and at equilibrium. Draw a figure showing this process.
Click here to check answer. click
A A
A p A
p
_
_
| p p p
| A
| p A p
| A
| A p A
|A
| p p p
| p
| A p p
|
|___________|
1. Is this an active or passive process?
Press here to check answer. press
2. Initially is there a diffusion gradient?
Press here to check answer. press
3. Initially which direction do the perfume molecules move?
- air molecules?
Press here to check answer. press
Draw a figure showing the arrangement of these gas molecules at equilibrium.
Check your figure to the figure below.
p A
A p A
p
_
_
| p p A
| A
| p A p
| p
| A p A
|A
| p A p
| p
| A p A
|
|___________|
Note that the lid is open and that the concentration of air "A"
molecules is equal to the concentration of perfume molecules "P".
4. At equilibrium which direction does net movement of perfume
molecules occur?
in, out of the
bottle, not at all (explain)
Press here to check answer. press
5. At equilibrium where is the higher concentration of perfume
molecules? (in the bottle, in the
room, same)
Press here to check answer. press
6. At equilibrium where is the greater number of perfume molecules? (in the bottle, in the room, same)
Press here to check answer. press
7. Does diffusion stop at equilibrium?
Press here to check answer. press
Diffusion is a passive phenomena where molecules randomly move
from one area of space to
another area down a gradient. There is no membrane barrier involved
during diffusion. The energy
that drives diffusion is not biological energy but energy acquired
from the environment as heat.
Osmosis - Passive Transport of Water Across a Membrane
What would happen if a selectively permeable membrane separated the two systems undergoing diffusion. Selectively permeable refers to a membrane which allows only certain molecules pass through.
Cells contain membranes that molecules must pass through. These membranes
are selectively
permeable because they allow some molecules pass through more rapidly
than others due to the
properties of the molecules and the membrane. In this section you will
learn how various
molecules pass across the membrane by osmosis, facilitated diffusion,
and active transport. Read
the pages indicated by B-19
and study behavioral
objective 18 .
Osmosis is the diffusion of water across a differentially
membrane. Study the figures in the reading demonstrating osmosis
and answer questions concerning the following situations.
Situation 1:
A differentially permeable membrane bag containing 10% sucrose solution (10 g sucrose/90 g water) is placed in a beaker containing 100% water. The bag in impermeable to sucrose but allows water to pass through.
8. Will water move out of the bag through the membrane?
Press here to check answer. press
9. Will water move into the bag through the membrane?
Press here to check answer. press
10. In which direction will most of the water move (into the bag
or out of the bag)? This is
called net movement.
Press here to check answer. press
11. Will sucrose move out of the bag through the membrane?
Press here to check answer. press
12. Will the solution in the bag ever reach equilibrium?
Explain. (equilibrium = no net movement of
water in this example)
Press here to check answer. press
13. Is water still moving out of the bag at equilibrium?
Press here to check answer. press
14. If membrane the does not burst will a pressure form inside
the bag? Define osmotic
pressure in your notes.
Press here to check answer. press
Examine the figures below representing situation one described above: initially and at equilibrium.
Selective permeable membrane bag initially with 10 %
sucrose place in a beaker containing water.
W = water molecules; s = sucrose molecules
W W W
W W W
W
____________
| W s s W | W
| W W W |
W
| W s s
| W
| W sW s | W
| s W W s |
W
|__________ | W
W W W
W
INITIAL
------------------------------------------------
W W W
W W W
W
____________
| W s W W | W
| s W s W |
W
| W s s W | W
| W sW s W | W
| s W W W |
W
|___________ | W
W W W
W
EQUILIBRIUM
Note: The number of sucrose molecules remained the same in the bag (8) but the number of water molecules increased; therefore, the concentration of sucrose in the bag decreases to less than 10 %. Because there is more water in the bag there must be an increase in pressure on the membrane. Observe the tight bag (turgor pressure, turgid, osmotic pressure).
Draw these figures in your notes and click here to go back and review
the questions concerning situation one. Click here to see questions concerning
situation one. click
Situation 2:
A differentially permeable membrane bag containing 100% water
is placed in a beaker
containing 10% sucrose (10 g sucrose dissolved in 90g water) . The
bag in impermeable to
sucrose but not water.
15. Will water move out of the bag through the membrane? Concentration gradient
Press here to check answer. press
16. Will water move into the bag through the membrane?
Press here to check answer. press
17. In which direction will most of the water move (into the bag
or out of the bag)?
This is called net movement.
Press here to check answer. press
18. Will sucrose move out of the bag through the membrane?
Press here to check answer. press
19. Will the solution in the bag ever reach equilibrium?
Explain equilibrium. No net movement
of water.
Press here to check answer. press
20. Is water still moving out of the bag at equilibrium?
Press here to check answer. press
21. If the membrane does not burst will a pressure form inside
the bag?
Press here to check answer. press
Examine the figures below representing situation two described above: initially and at equilibrium.
Selective permeable membrane bag, initially with water
placed in a beaker containing 10% sucrose solution..
W = water molecules; s = sucrose molecules
W s s
W s W
W
____________
| W W W | s
| W W W |
W
| W W W | W s
| W W W | s
W
| W W W |
W
|__________ | W
W s W W
s
INITIAL
-----------------------------------------------------------------------------------------------------------------------
W W s W
W W s W s
W
____________
| W W W | W s
| W W
| W
| W W
| W s
| W
W | W
| W W
| s W
|____________| W
W s W s W
W
EQUILIBRIUM
Note: The number of sucrose molecules remained the same outside the bag (8). The number of water molecules outside the bag increases; therefore, the concentration of sucrose in the bowl decreases to less than 10 %. Because there is less water in the bag there must be a decrease in the pressure on the membrane. (flaccid, negative osmotic pressure, plasmolysis)
Draw these figures in your notes and click here to go back and review
the questions concerning situation two. Click here to see
questions concerning situation two. click
Situation 3:
A differentially permeable membrane bag containing 30% sucrose solution
(30 g sucrose
dissolved in 70 g water) is placed in a beaker containing 10% sucrose
(10 g sucrose dissolved in
90 g water). The bag in impermeable to sucrose but not water.
22. Will water move out of the bag through the membrane? Concentration gradient
Press here to check answer. press
23. Will water move into the bag through the membrane?
Press here to check answer. press
24. In which direction will most of the water move (into the bag
or out of the bag)? This is
called net movement.
Press here to check answer. press
25. Will sucrose move out of the bag through the membrane?
Press here to check answer. press
26. Will the solution in the bag ever reach equilibrium?
Press here to check answer. press
27. Is water still moving out of the bag at equilibrium?
Press here to check answer. press
28. If the membrane does not burst will a pressure form inside
the bag?
Press here to check answer. press
Examine the figures below representing situation three described above: initially and at equilibrium.
Selective permeable membrane bag, initially with 30% sucrose
placed in a beaker containing 10% sucrose solution..
W = water molecules; s = sucrose molecules
W s W
W W W
W
____________
| W s W s | s
| s W s
| W
| W W s W | W s
| s s W
| W W
| Ws W s |
W
|__________ | W
W s W W
s
INITIAL
----------------------------------------------------------------------------
W W s W
W W W
W
____________
| WW sWW | W s
| s W s W |
W
|s W W s W | s
| W s W W W| W
| s W s W s | s
W
|___________| W
W W s W
W
EQUILIBRIUM
Note: The number of sucrose molecules remains the same inside and outside the bag (8-5). However the concentration of sucrose will change. The water molecules move from the bowl into the bag (water moving from a high to low concentration. Since water molecules were moving into the bag from the bowl the concentration of sucrose in the bag decrease and the concentration of sucrose in the bowl increase. This will continue until the concentration of sucrose in the bag and in the bowl are the same. At this point water will move into and out of the bag at the same rate; thus, equilibrium will be established.
Draw these figures in your notes and click here to go back and review
the questions concerning situation three.
Click here to go back to questions concerning question
three. click
Think and Explain: During diffusion of gases the concentration
of particles will be the same at all points in the container and the room.
During osmosis the concentration of particles may or may not be the same
on both sides of the membrane.
Solutions: hypotonic, hypertonic, isotonic
Read the section on solutions in the readings form B-19 and study behavioral objective 20. Solutions consists of two parts - solutes and solvents. Solvent is a liquid capable of dissolving other substances in itself. The substance being dissolved is called a solute. In the above examples water is the solvent and sucrose is the solute.
Solutions can be classified as hypertonic (hyperosmotic), hypotonic (hyposmotic) or isotonic (isosmotic). Write in your notes the definition for these three terms.
Note: You must compare at least two solutions when using these terms.
Fill in the chart below to describe these three solution types.
| Solution Type | Amount of Solute (greater, lesser, same) | Amount of Solvent (greater, lesser, same) |
| Hypotonic | ||
| Hypertonic | ||
| Isotonic |
29. A sugar solution contains 15g glucose dissolved in 85 g water.
Is this solution hypertonic,
isotonic, or hypotonic?
Press here to check answer. press
30-35. Match the following terms to the correct statement listed below.
a) isotonic
b) hypertonic
c) hypotonic
30. A solution with a higher concentration of solute molecules compared to a second solution.
a) isotonic
b) hypertonic
c) hypotonic
Press here to check answer. press
31. A solution with a lower concentration of solute
molecules compared to a second solution.
a) isotonic
b) hypertonic
c) hypotonic
Press here to check answer. press
32. A solution with the same concentration
of solute molecules as the second solution.
a) isotonic
b) hypertonic
c) hypotonic
Press here to check answer. press
33. In situation 3 above the solution in the bag is ________
compared to the solution in the
beaker. Check notes to see
concentrations in the bag and beaker.
a) isotonic
b) hypertonic
c) hypotonic
Press here to check answer. press
34. The solution of the bag in situation 1 is _________
compared to the solution of the bag in
situation 2. Check your
notes to see concentrations is the bag and beaker.
a) isotonic
b) hypertonic
c) hypotonic
Press here to check answer. press
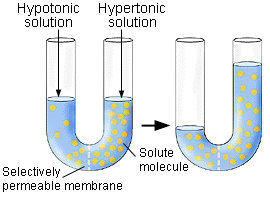
The U tube above shows a hypotonic and hypertonic solution separated
by a selectively permeable membrane. Note the pressure that builds
up on the hypertonic side. This is the osmotic pressure.
35. If a bag contains a hypertonic solution compared to the solution
in the beaker, the bag will
__________ (swell up, shrink,
remain the same).
Press here to check answer. press
Osmosis: Transport of Water into Living Systems
Now let us study osmosis in plant and animal cells..
The cell membrane is a selective permeable membrane. The solution of
the cell's cytoplasm will
react the same way the bags did in the above situations. For example
study the figure in the
readings (B-19)
showing the effect of osmosis on red blood cells.
36. A red blood cell placed in a hypotonic solution
will __________ (swell up, shrink, remain
the same). Since the
cell membrane is not strong it will break releasing the cytoplasm
contents to the outside.
This is called hemolysis. Lysis means to break.
Press here to check answer. press
37. A red blood cell placed in an isotonic solution
will __________ (swell up, shrink, remain the
same).
Press here to check answer. press
38. A red blood cell placed in a hypertonic solution will
__________ (swell up, shrink, remain
the same). This is called
crenation. If left in this condition very long the cell will die.
Press here to check answer. press
Explain what would occur if a plant cell was placed in these three conditions.
39. A plant cell placed in a hypotonic solution will __________ (swell up, shrink, remain the same).
Press here to check answer. press
However the plant cell will not break due to the presence of a cell
wall. The cell wall will become very tight due to the osmotic pressure.
This pressure is called turgor pressure when plant cells are involved
and the cell is described as being turgid.
40. A plant cell placed in a hypertonic solution will __________ (swell up, shrink, remain the same).
Press here to check answer. press
The cytoplasm will become separated from the cell wall (the cell wall does not shrink). This is called plasmolysis. The cell in this state is said to be flaccid.
In your notes draw figures showing hemolysis, crenation, plasmolysis,
turgid cell and write a
paragraph explaining how these were formed by osmosis.
41. Plant cells of a wilted plant are in what condition? (plasmolyzed, turgid, crenated, hemolyzed)
Press here to check answer. press
o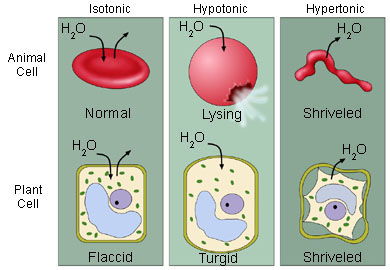
Draw these animal and plant cells in your notes with the proper terms.
42. Is osmosis an active or passive process? Explain
Press here to check answer.
press
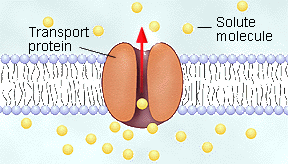
Facilitated Diffusion: Transport of Ions and Small Molecules Across Membrane
Facilitated Diffusion: Read the pages indicated by B-19 and study behavioral objectives 18 . In your notes write definition for facilitative diffusion. Facilitated diffusion is exactly like osmosis except the molecules must use membrane proteins to be transferred across the cell membrane. This will allow for selectivity as to which molecules can pass through and allows for polar and larger molecules to pass through the membrane.
Osmosis is the movement of water across the membrane. Other molecules
such potassium ions,
calcium ions, amino acids, and monosaccharides can only move across
the membrane with the aid
of channel proteins or carrier proteins. In your notes draw
figures taken from the text and
describe how membrane proteins help transport these
molecules. This type of transport will only
move molecules down their concentration gradients similar to osmosis
(from a high concentration to a low concentration).
Identify the transport molecule "c" and the glucose (solute molecules).
Membrane with carrier molecules "c" which will only transport specific
molecules such as glucose "g". The solution inside the cells consists
of 10% ionic salt. The solution in the bowl contains 6% glucose (6) and
5% sucrose (5).
W = water molecules; s = sucrose molecules; I = inorganic salts;
g = glucose molecules
W s g W
W W g W
W
___c____c___
| W I W I | s
| I W I
| g W
| W W I W | W s
| I I W
c W W
| WI W I |
g W
|_____c_____ c W
W g s W g W
s
INITIAL
-----------------------------------------------------------------------------------------------------------------------
W W s W
W W g W
W
___c_____c__
| WW IWW | W s
| I W I g
| W
|I g W I W |
s
| W I g W W c W
| I W I W I | s
g
|_____c_____c W
W W s W
g
EQUILIBRIUM
Note: The glucose molecules moved into the bag until
the concentration of glucose in the bag was the same as glucose outside
of the bag (both the solutions inside and outside contain 3% glucose (3)).
The sucrose molecules and the inorganic ions did not move across
the membrane. Why?
Read the pages indicated by B-20
which give examples of these type of transport in humans.
43. Carrier molecules located in the membrane will allow all small molecules
to pass into the
cytoplasm?
Press here to check answer.
press
Active Transport: Transport of Molecules Across Membrane
Actively
Active Transport: Read in the text the pages indicated by B-19
and study behavioral
objective 19. Active transport is similar to facilitative transport
(uses carrier membrane protein) except biological energy ATP will be required
for the carrier protein to operate. Also the carrier molecules will
allow selective transport in one direction which will allow molecules to
move against a concentration gradient - low concentration to a high
concentration. Study the figure below.
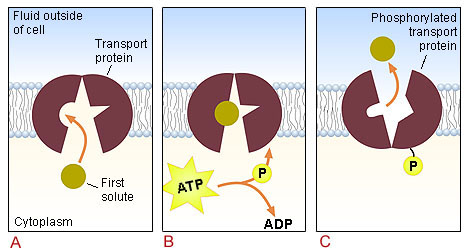
Membrane with energy driven carrier molecules "ce" which will only transport
specific molecules such as glucose "g" into the cell. The solution
inside the cells consists of 10% ionic salt. The solution in the bowl contains
6% glucose (6) and 5% sucrose (5).
W = water molecules; s = sucrose molecules; I = inorganic salts;
g = glucose molecules
W s g W
W W g W
W
___ce____ce___
| W I W I
| s
| I W I
| g W
| W W I W | W
s
| I I W
ce W W
| W I W I
| g W
|_____ce______ ce W
W g s W g W
s
INITIAL
-----------------------------------------------------------------------------------------------------------------------
W W s W
W W s W
W
___ce_____ce__
| W W I W W | W s
| I g W I g
| W
|I g W I W
| s
| W I g W W ce W
| I g W g I W I | s
W
|_____ce_______ce W
W W s W
EQUILIBRIUM
Note: All of the glucose molecules moved into the bag.
The sucrose molecules and the inorganic ions did not move across
the membrane. Did the glucose molecules move against a concentration
gradient?
In your notes write a paragraph comparing facilitative diffusion
with active transport. Contrast active transport with facilatative transport
as to use of biological energy and type of concentration
gradient.
Click here to learn how these concepts are used in the human respiratory
system.
' '
click
For information on how to use this page, go to How
to Use This Site.
Created by the Center for Learning Technologies, Academic Technology Services.
Last modified October 22, 1997.